High performance liquid chromatography analysis method for separating and determining L-alanine isopropyl ester hydrochloride enantiomer
A technology of isopropyl alanine and high performance liquid chromatography, applied in the field of separation and determination of enantiomers of L-alanine isopropyl hydrochloride, high performance liquid chromatography, can solve the problem of being sensitive to other substances Interference, difficult control of derivatization time, poor solution stability and other problems, to achieve the effect of short analysis time, good peak shape symmetry and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
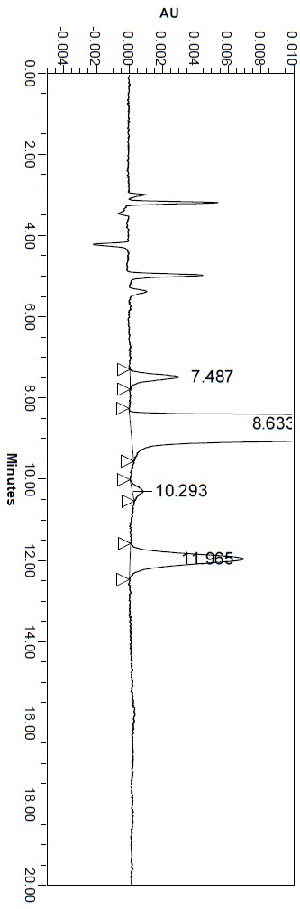
[0034] Example 1 Selection of chromatographic conditions
[0035] 1.1 The choice of wavelength
[0036] Mobile phase: n-hexane-absolute ethanol-diethylamine ratio is 80:20:0.1.
[0037] Preparation of D-alanine isopropyl hydrochloride derivative solution: Weigh about 10 mg of D-alanine isopropyl hydrochloride derivative, put it in a 10 ml measuring flask, add mobile phase as a diluent to dissolve, And diluted to the mark, shake well, that is.
[0038] Take the D-alanine isopropyl ester hydrochloride derivative solution as the test sample, use the mobile phase as the blank calibration solution, and scan at 190nm~400nm according to the ultraviolet-visible spectrophotometry (Chinese Pharmacopoeia 2020 edition). The results show that the test solution has a maximum absorption peak at 225nm. Therefore, 225nm±5nm was determined as the detection wavelength.
[0039] 1.2 Durability test of the method
[0040] In this test, the chromatographic method of this test is screened by ad...
Embodiment 2
[0052] Example 2 Stability of the solution
[0053] Chromatographic conditions: Same as scheme 1 of Example 1.
[0054] Preparation of the solution:
[0055] Need testing solution: prepare according to the same method of 100% concentration level need testing solution in embodiment 1;
[0056] Control solution: Precisely pipette 1ml of the test solution, put it in a 100ml measuring bottle, dilute it to the mark with diluent, shake well, then precisely pipette 1ml, put it in a 20ml measuring bottle, dilute to the mark with mobile phase as a diluent, and shake well , filtered with a 0.22 μm organic filter, and the subsequent filtrate was obtained.
[0057] At 0 hours, 4 hours, 8 hours, 12 hours, and 24 hours, 10 μl of the test solution and the control solution were accurately measured and tested, and the chromatograms at different time points were recorded. The results are shown in Table 3.
[0058] Table 3 Solution stability results
[0059]
[0060] The results showed t...
Embodiment 3
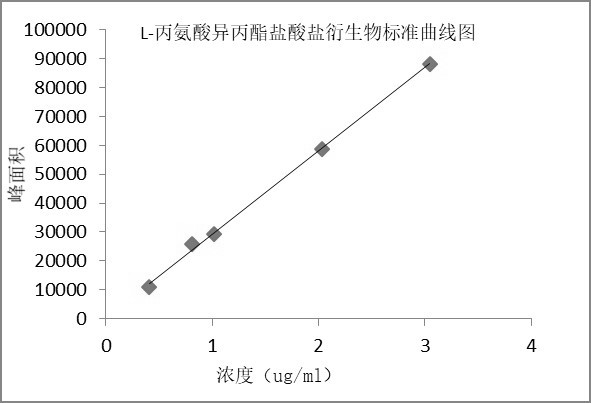
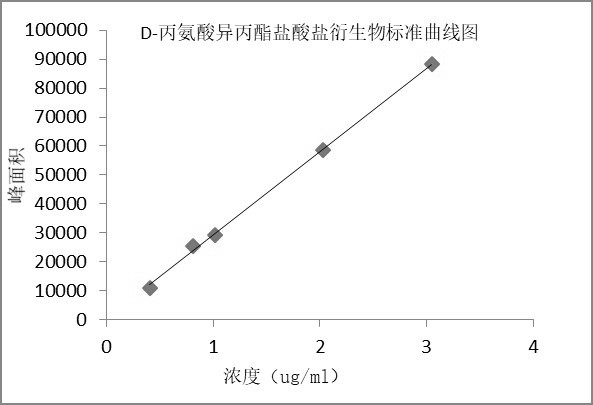
[0061] Example 3 Preparation of standard curve
[0062] Chromatographic conditions: Same as scheme 1 of Example 1.
[0063] Preparation of the solution:
[0064] Linear stock solution: take about 20 mg of L-alanine isopropyl ester hydrochloride derivative and D-alanine isopropyl ester hydrochloride derivative reference substance, respectively, put them in 10 ml measuring flasks, and add mobile phase as Dissolve the diluent and dilute it to the mark, shake well; precisely measure 1ml each, put it in the same 100ml measuring bottle, dilute it to the mark with mobile phase, and shake well to get it;
[0065] Standard curve solution: Dilute with mobile phase linear stock solution to prepare solutions of L-alanine isopropyl hydrochloride derivatives and D-alanine isopropyl hydrochloride derivatives at different concentrations, see Table 4 :
[0066] Table 4 Preparation of standard curve solution
[0067]
[0068] The results showed that the L-alanine isopropyl ester hydrochl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com