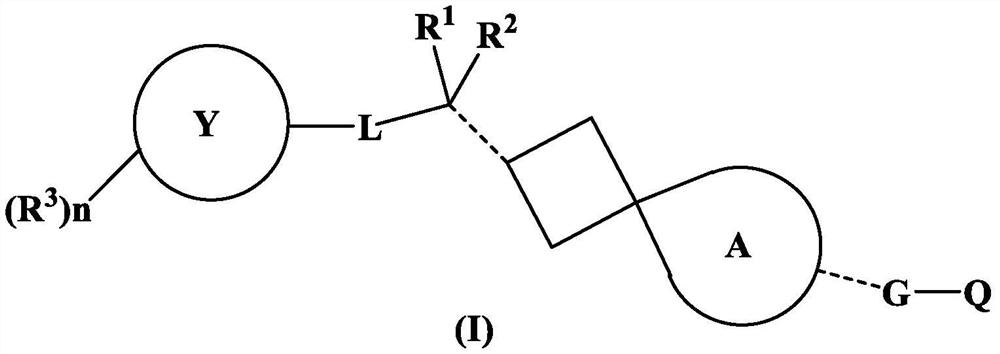
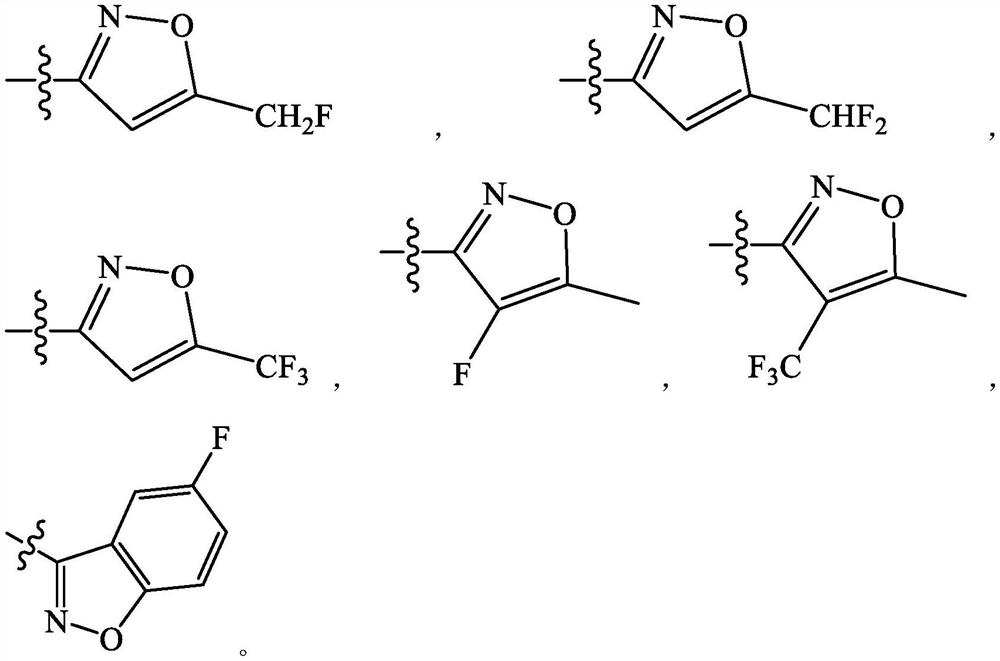
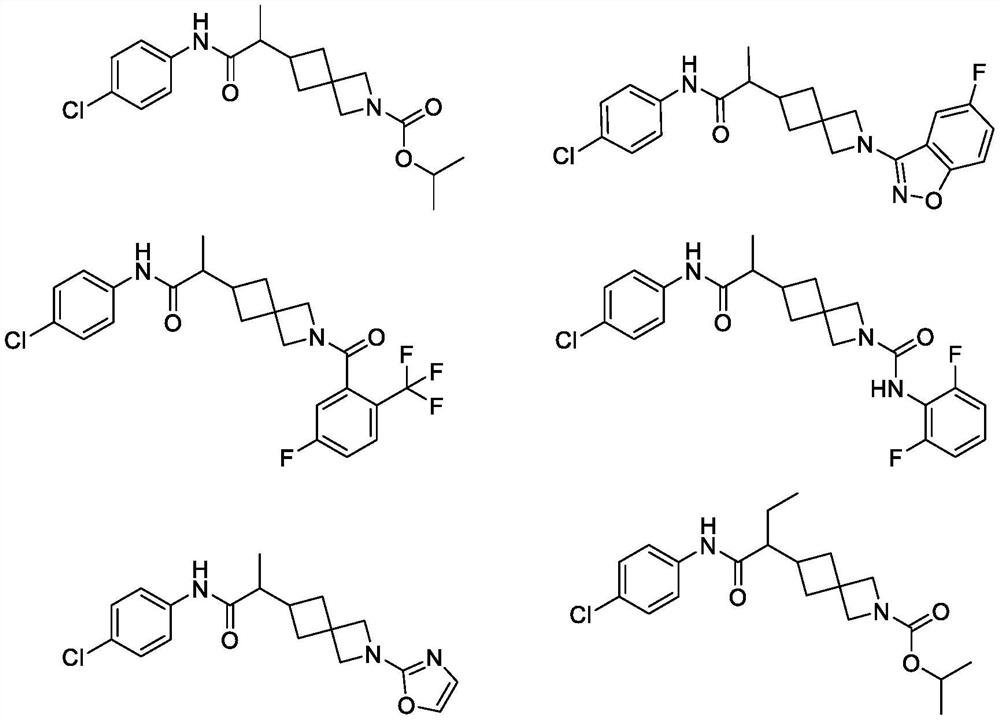
Spiro compound as indoleamine 2, 3-dioxygenase inhibitor
A compound, solvate technology, applied in the treatment of proliferative diseases, immune-related diseases and/or inflammatory diseases, infectious diseases, and can solve the problem of ineffective immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Embodiment 1 (synthesis method 1)
[0124] (S or R)-6-(1-((4-Chlorophenyl)amino)-1-oxopropan-2-yl)-2-azaspiro[3.3]heptane-2-carboxylate isopropyl Ester 1a; (R or S)-6-(1-((4-chlorophenyl)amino)-1-oxopropan-2-yl)-2-azaspiro[3.3]heptane-2-carboxy Isopropyl acid 1b
[0125]
[0126] step 1
[0127]
[0128] To a stirred solution of ethyl 2-(diethoxyphosphoryl)propionate (6.8 g, 28.4 mmol, 1.2 equiv) in dry tetrahydrofuran (68 Sodium hydride (1.9 g, 47.3 mmol, 2.0 equiv, 60% mixture in mineral oil) was added. The mixture was warmed to room temperature and stirred at the same temperature for 15 minutes; then to it was added tert-butyl 6-oxo-2-azaspiro[3.3]heptane-2-carboxylate (5 g, 23.7 mmol, 1.0 equiv) in dry tetrahydrofuran (50 mL). The reaction was stirred under nitrogen protection at room temperature for 3 hours, and the reaction process was monitored by liquid mass and thin layer chromatography. After the reaction was completed, the reaction solution was co...
Embodiment 2
[0149] Example 2 (Synthetic Method II)
[0150] (S or R)-N-(4-chlorophenyl)-2-(2-(5-fluorobenzo[d]isoxazol-3-yl)-2-azaspiro[3.3]heptane- 6-yl)propionamide 2a; (R or S)-N-(4-chlorophenyl)-2-(2-(5-fluorobenzo[d]isoxazol-3-yl)-2-nitrogen Heterospiro[3.3]heptan-6-yl)propionamide 2b
[0151]
[0152] step 1
[0153]
[0154] To compound 1-5 (193.59 mg, 0.69 mmol, 1.50 equiv) and 3-chloro-5-fluoro-benzo[d]isoxazole (100 mg, 0.46 mmol, 1 equiv) in N,N-dimethylacetamide (5 mL) was added cesium carbonate (301.67 mg, 0.93 mmol, 2.0 equiv). The resulting mixture was stirred at 80 degrees Celsius for 16 hours. The progress of the reaction was monitored by liquid mass and thin layer chromatography. After the reaction, the reaction mixture was directly purified by reverse phase chromatography (C18 column): eluted with 50%→70% acetonitrile / water within 10 minutes; detector: UV 254 nm; compound 2-1 (white solid) was obtained. , 50 mg, 21% yield). MS(ESI,m / z):414.1 / 416.1[M+H] + ....
Embodiment 3
[0160] Embodiment 3 (synthesis method III)
[0161] (R or S)-N-(4-chlorophenyl)-2-(2-(5-fluoro-2-(trifluoromethyl)benzoyl)-2-azaspiro[3.3]heptane- 6-yl)propionamide 3a; (S or R)-N-(4-chlorophenyl)-2-(2-(5-fluoro-2-(trifluoromethyl)benzoyl)-2-nitrogen Heterospiro[3.3]heptan-6-yl)propionamide 3b
[0162]
[0163] step 1
[0164]
[0165] At room temperature, 5-fluoro-2-(trifluoromethyl)benzoic acid (89.6 mg, 0.43 mmol, 1.2 equiv) and 2-(7-azobenzotriazole)-N,N,N ',N'-Tetramethylurea hexafluorophosphate (191.0 mg, 0.50 mmol, 1.4 equiv) was dissolved in N,N-dimethylformamide (1 mL). The resulting mixture was stirred at room temperature for 15 minutes and then compound 1-5 (100 mg, 0.36 mmol, 1.0 equiv.) and diisopropylethylamine (129.8 mg, 1.0 mmol, 2.8 equiv.) were added sequentially, followed by continuing at room temperature. The reaction was carried out for 3 hours. The reaction process was monitored by liquid mass and thin-layer chromatography. After the reaction, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com