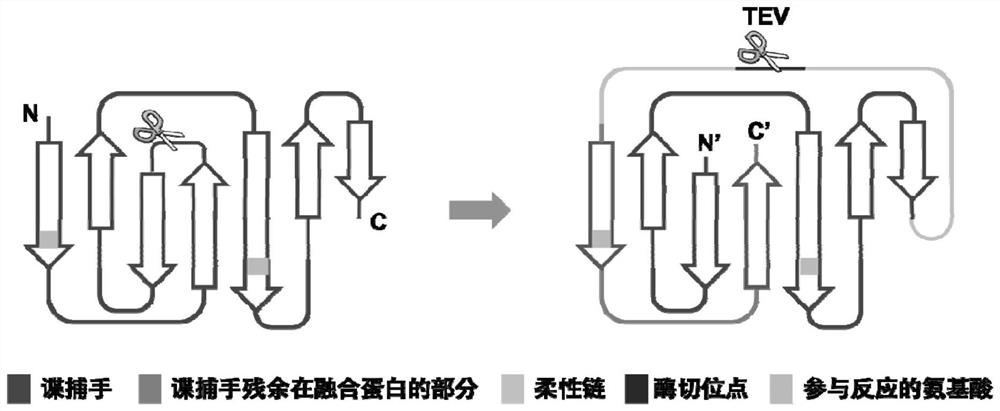
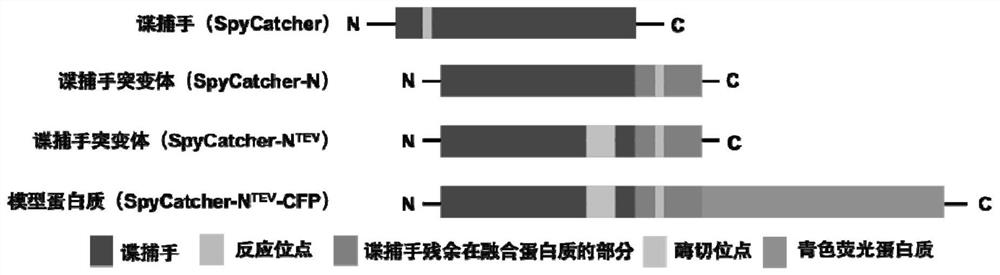
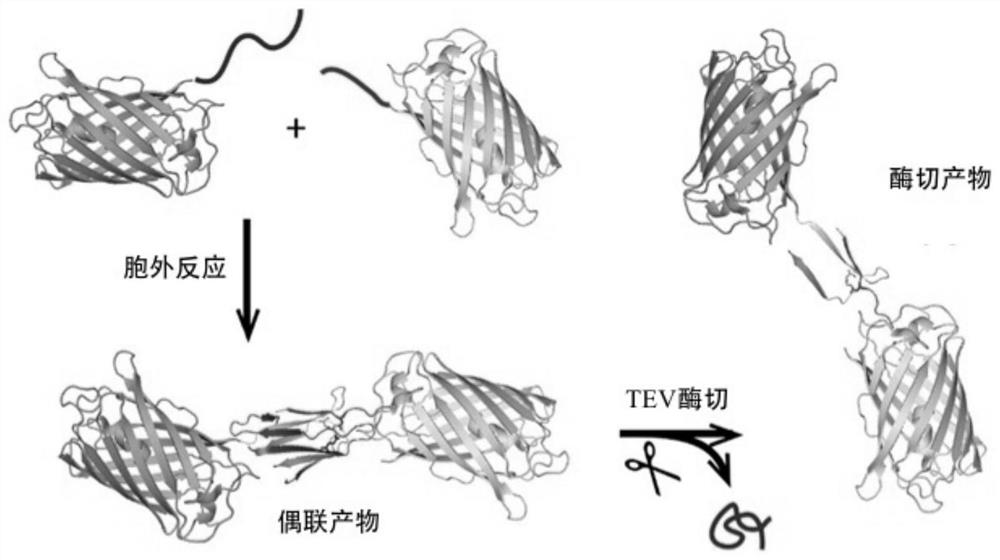
Syy capturer mutant, preparation method thereof and application of spy capturer mutant in fluorescent protein system
A mutant and protein technology, applied in the fluorescent protein system, spy catcher mutant and its preparation field, can solve the problems of reactivity and other problems, and achieve the effect of reducing the molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The genetic design is as follows:
[0026] The selected model proteins, yellow fluorescent protein and cyan fluorescent protein, are both derived from green fluorescent protein. The 203rd threonine (T203) of green fluorescent protein is mutated to tyrosine to obtain yellow fluorescent protein, and the side group of tyrosine interacts with the π-π interaction of the chromophore to make the excitation wavelength and emission wavelength of the fluorescent protein red. The excitation wavelength is 517 nm and the emission wavelength is 530 nm. The 66th tyrosine (Y66) of green fluorescent protein is mutated to tryptophan to obtain cyan fluorescent protein. The side group of tryptophan makes the chromophore finally form an indole ring instead of a phenol compound. The excitation wavelength is 435 nm and the emission wavelength is 477nm.
[0027] In addition to re-splitting the original protein domain to obtain smaller linkers, circular permutation of the protein structure an...
Embodiment 2
[0030] Plasmids were constructed as follows:
[0031] For Exploring Spy Tags and Spy Catcher-N TEV In the application in the model system, choose yellow fluorescent protein and cyan fluorescent protein as model proteins, construct spy tag-yellow fluorescent protein (SpyTag-YFP) and spy catcher-N TEV- Cyan fluorescent protein (BDTag-CFP). Taking the construction of the spy tag-yellow fluorescent protein sequence as an example, using the plasmid containing the yellow fluorescent protein sequence as the template, and the DNA single-stranded fragment containing the restriction enzyme cleavage site sequence as the primer, PCR was used to amplify the spy tag containing the restriction enzyme cleavage site- Sequence of yellow fluorescent protein of interest. Then, the spy tag-yellow fluorescent protein fragment was inserted into the pET-28b vector containing the same restriction site by the method of double digestion. Spy Catcher-N TEV - Cyan fluorescent protein (BDTag-CFP) was c...
Embodiment 3
[0039] Protein purification and expression
[0040] Contains Spy Tag-Yellow Fluorescent Protein (SpyTag-YFP) and Spy Catcher-N TEV - After the pET-28b plasmid of cyan fluorescent protein (BDTag-CFP) was confirmed by sequencing, it was transferred into BL21 (DE3) competent cells and plated, and cultured in an incubator overnight at 37°C. Pick out monoclonal colonies on the plate into LB medium containing 0.33 mg / mL kanamycin, and cultivate overnight at 37°C in a shaking incubator. Take the medium from the overnight culture and add it to fresh 1L LB medium containing 0.33mg / mL kanamycin sulfate at a ratio of 1:100, and shake at 37°C to culture to OD. 600 To 0.4-0.6, the temperature of the shaking incubator was changed to 16 °C for overnight culture, and IPTG was added to the final concentration of 1 mM to induce E. coli to express the target protein. After the expression, the bacteria were harvested by centrifugation and the supernatant was discarded, and the target protein wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com