Lactobacillus paracasei and application thereof in prevention of streptococcus infection of infants
A technology of Lactobacillus and paracheese, which is applied to medical preparations containing active ingredients, bacteria, antibacterial drugs, etc., can solve problems such as no necrotizing enterocolitis or septicemia prevention effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
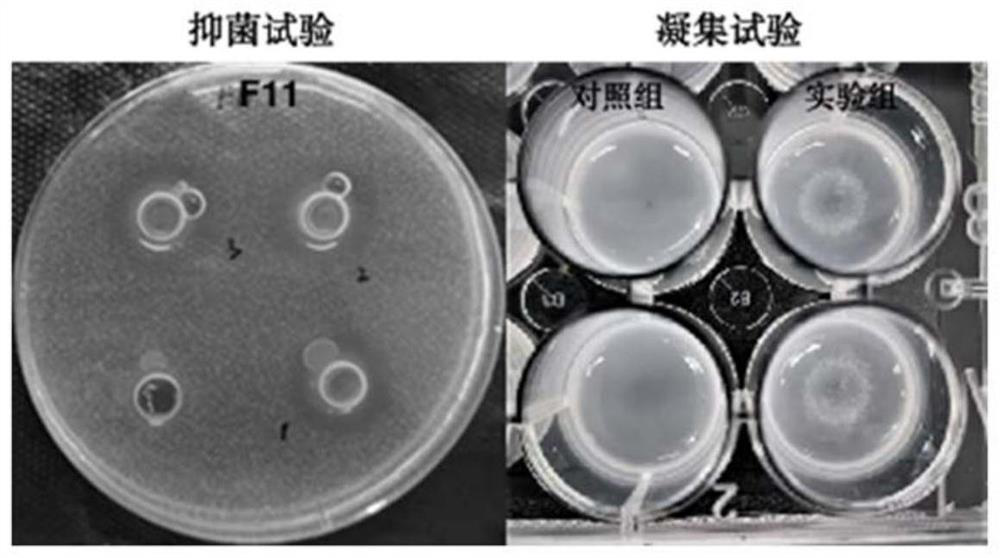
[0028] Example 1 Screening of Lactobacillus paracasei VHProbi F11
[0029] 1. Bacteria isolation
[0030] Prepare MRS (Man Rogosa Sharpe) broth: pure water 1L, peptone 10g, beef extract 10g, yeast extract 5.0g, sodium acetate 5g, glucose 5g, potassium dihydrogen phosphate 2g, Tween 80 1.0mL, citric acid Diamine 2.0g, calcium carbonate 20g, magnesium sulfate heptahydrate 0.58g, manganese sulfate heptahydrate 0.25g, adjusted pH 6.2-6.5.
[0031] Configure MRS agar medium: add 15g agar to 1LMRS broth.
[0032] According to the 2019 version of the "Human Genetic Resource Bank Ethics Code", after signing the project commitment letter and informed consent with the sample provider, in accordance with the standard operating specifications of the biobank, take 1g of fresh fecal samples from healthy infants who have not consumed probiotic preparations within half a year. , diluted with sterile physiological saline, put it into a sterile sample bag, beat and mixed with a homogenizer; t...
Embodiment 2
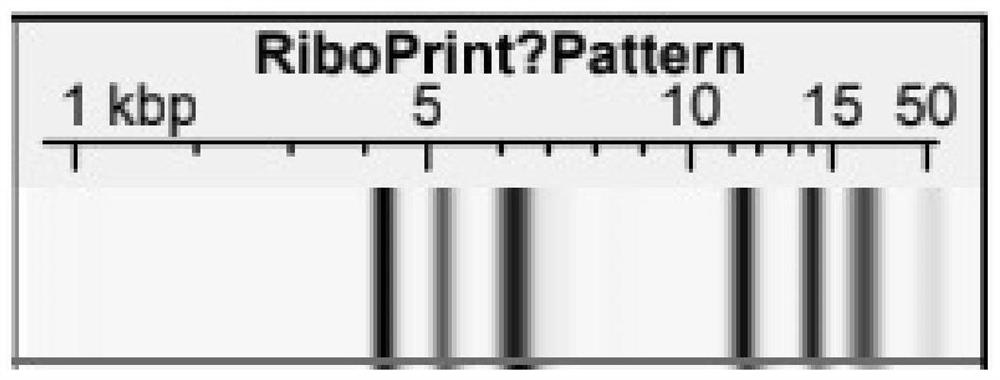
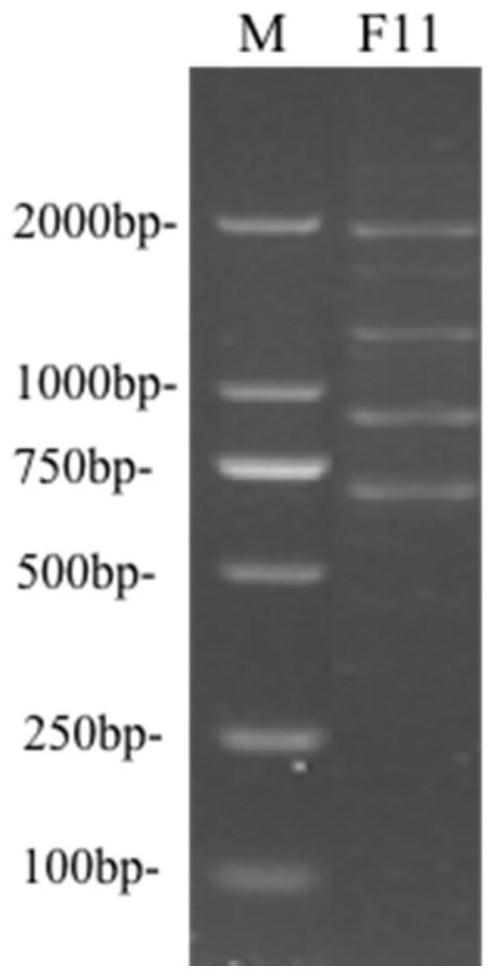
[0042] Example 2 Identification of F11 strain
[0043] 1. Identification of colony morphology
[0044] The F11 strain was inoculated on the MRS agar medium, and after anaerobic culture at 37°C for 24 hours, the single colony of F11 was milky white, the colony diameter was about 1-2mm, the surface was smooth, the edges were round and convex, and the strain under the microscope was bacilli. .
[0045] 2. Identification of physiological and biochemical characteristics
[0046] The preparation of the inoculum in this example is as follows: under aseptic conditions, take an appropriate amount of fresh F11 bacterial solution, centrifuge at 5000 rpm / min for 5 min, wash twice with PBS buffer, and then reconstitute the bacteria with the same volume of PBS buffer and dilute 50 times as the inoculum.
[0047] 2.1. Salinity tolerance test
[0048] Under sterile conditions, add 190 μL of BSM liquid medium with a salt concentration of 1%, 2%, 3%, 4%, 5%, 6%, 7%, and 8% to a 96-well plat...
Embodiment 3
[0104] Example 3 Tolerance test of Lactobacillus paracasei VHProbi F11 to artificial gastric juice
[0105] 1. Preparation of artificial gastric juice
[0106] Weigh 5 g of peptone, 2.5 g of yeast extract, 1 g of glucose and 2 g of NaCl, add 1000 mL of distilled water, adjust pH to 3.0 with dilute hydrochloric acid, and then sterilize at 115 °C for 20 min. Then add 3.2 g of porcine mucosal pepsin before use, shake well to dissolve, and place in a warm water bath for 1 hour in a 37°C water bath shaker to simulate human body temperature.
[0107] 2. Test method
[0108] Take 2 mL of fresh bacterial liquid, centrifuge at 5000 rpm / min for 5 min to collect the bacterial cells, wash the bacterial cells three times with normal saline, and resuspend with 2 mL of normal saline as the inoculum. Take 1 mL of inoculum, add it to 24 mL of artificial gastric juice, place it on a water bath shaker (200 rpm / min) at 37 °C for 3 h, and sample 1 mL to detect the amount of viable bacteria.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com