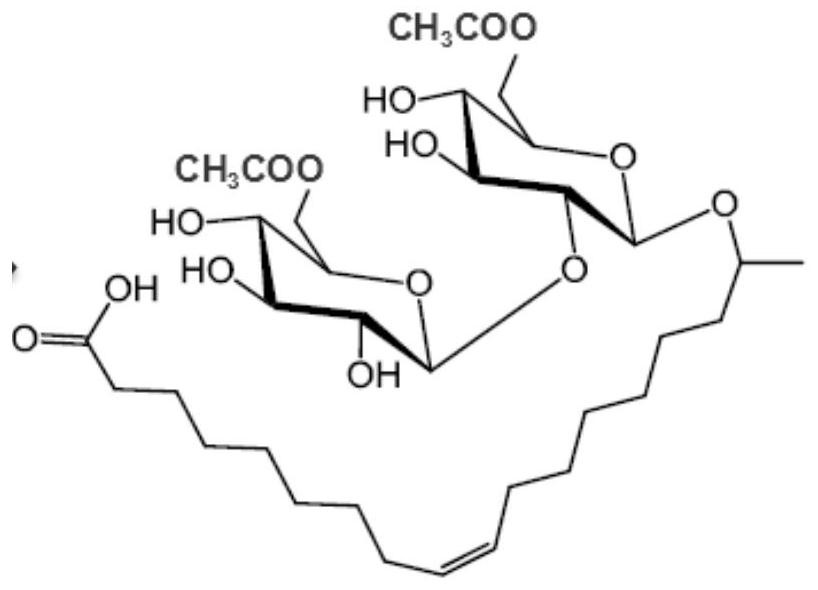
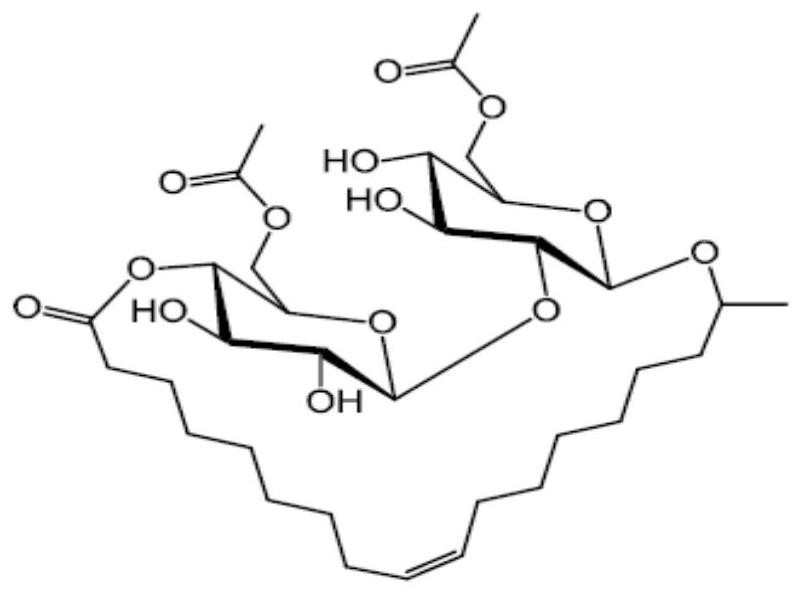
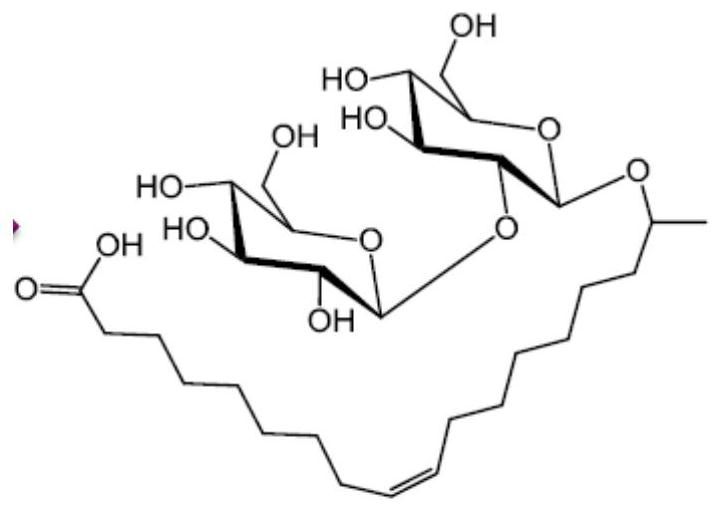
Sophorolipid deacetylation modification method and sophorolipid
A technology of deacetylation and sophorolipids, which is applied in the field of preparation of deacetylation modification of sophoryl esters, can solve problems affecting product appearance, color performance, chain breakage of fatty acid chains, affecting product performance, etc., to achieve low decolorization Cost, good chroma quality, effect of simple decolorization means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Deacetylation of Sophorolipids
[0026] Take 2g of dry sophorolipid powder and place it in a 100mL round-bottom flask. The round-bottom flask is connected with a condensing reflux tube (refrigerant temperature 10°C) and a protection tube with anhydrous calcium chloride to prevent the influence of moisture in the atmosphere. reaction. During the reaction, 25 mL of a methanol solution of 0.025 mol / L sodium methoxide was added, and a magnetic stirring reaction was carried out at room temperature of 25° C. for 5 hours to carry out an ester hydrolysis reaction. After that, 25 mL of pure water was added, and the reaction was further magnetically stirred for 1 hour. Then, the reaction solution was taken out, placed in a rotary evaporator and concentrated in vacuo at 35° C. to remove methanol in the recovered reaction system, thereby obtaining a deacetylated acid-type sophorose ester solution. The powder samples of sophorose esters were obtained from deacetylated a...
Embodiment 2
[0027] Example 2: Deacetylation of Sophorose Esters
[0028] Take 2g of dry sophorolipid powder and place it in a 100mL round-bottom flask. The round-bottom flask is connected with a condensing reflux tube (refrigerant temperature 10°C) and a protection tube with anhydrous calcium chloride to prevent the influence of moisture in the atmosphere. reaction. During the reaction, 25 mL of an ethanol solution of 0.02 mol / L sodium ethoxide was added, and a magnetic stirring reaction was carried out at 30° C. for 5 hours to carry out an ester hydrolysis reaction. Then, 25 mL of pure water was added, and the transesterification magnetic stirring reaction was carried out for 1 hour. Then, the reaction solution was taken out, placed in a rotary evaporator and concentrated in vacuo at 40° C. to remove the ethanol in the recovered reaction system, thereby obtaining a deacetylated acid-type sophorose ester solution. The powder samples of sophorose esters were obtained from deacetylated ac...
Embodiment 3
[0029] Example 3: Alkaline hydrolysis control reaction
[0030] With reference to the method described in Example 19 of the PCT patent application Improved Sopholactone Production (International Application Number: PCT / EP2011 / 059306), the deacetylated sophorolipids prepared by the alkaline hydrolysis method were prepared. The sophorolipid aqueous solution with a solid content of 50% was prepared by adding 3 times the molar equivalents of KOH and 2 times the water, after which the reaction mixture was heated to 50 °C, stirred for 10 minutes, and then passed through a glass fiber filter twice, and then the mixture was The reaction was heated to 80°C for 4 hours to complete the complete hydrolysis and obtain deacetylated sophorolipids. The pH of the reacted mixture was adjusted to pH 1.5 using 37% HCl and left to settle overnight. The acid sophorolipids were separated from the supernatant salt solution (KCl and potassium acetate) by centrifugation. The sophorolipids collected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com