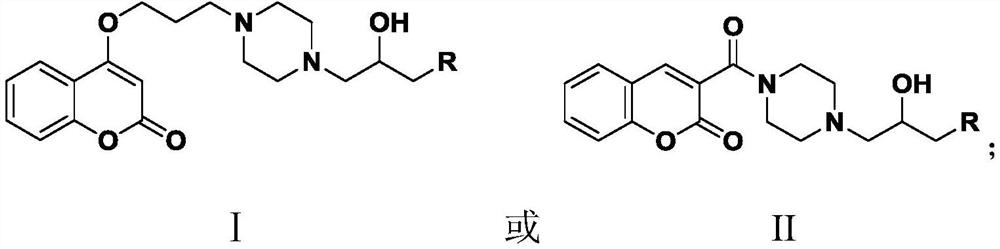
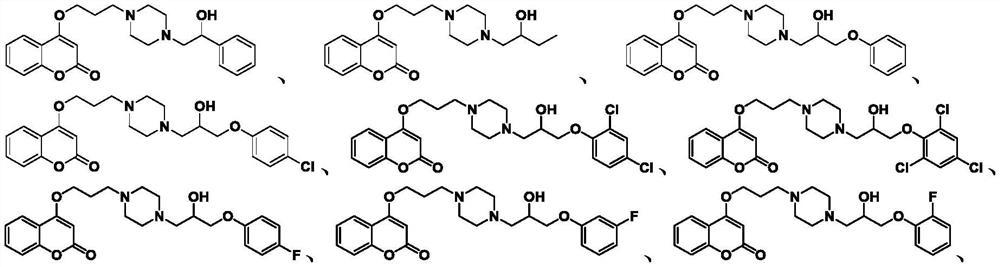
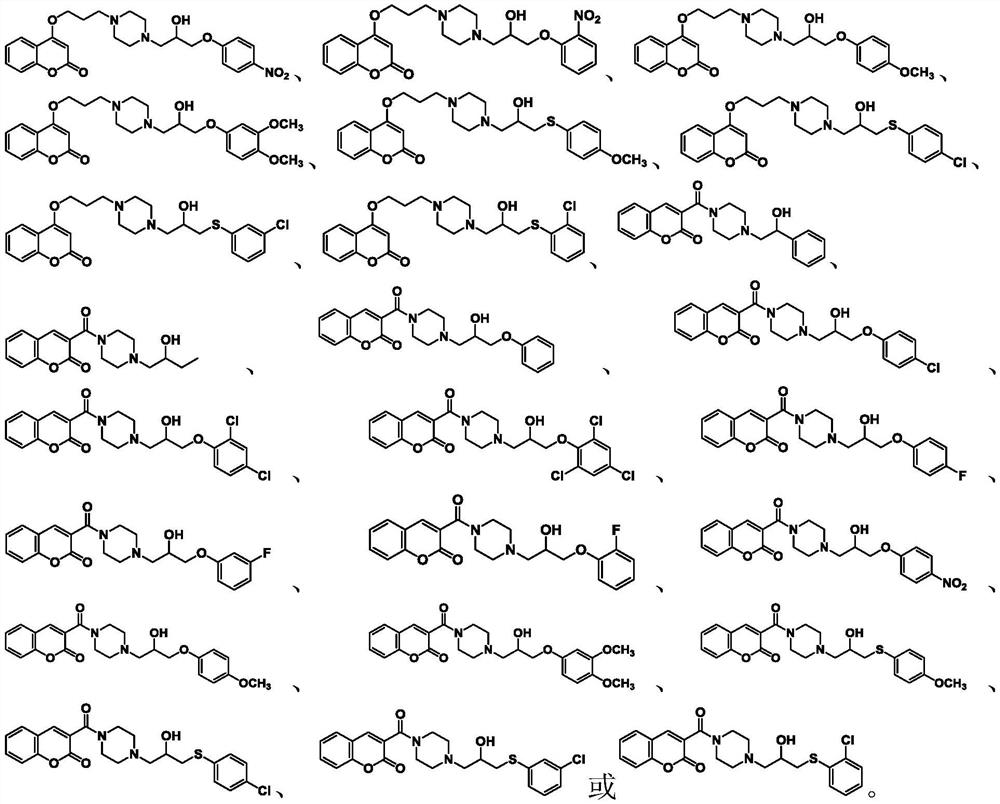Coumarin compound containing isopropanolamine structure as well as preparation method and application of coumarin compound
A technology of isopropanolamine and coumarins, which is applied in the field of coumarins and their preparation, can solve the problems of increasing the resistance of plant pathogenic microorganisms, endangering crop safety, affecting the environment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0057] Example 1-1: Preparation of 4-(3-bromopropanyl)-2H-benzopyran-2-one
[0058]
[0059] In a 100 mL round-bottomed flask, 4-hydroxycoumarin (18.51 mmol) and 30 mL of DMF were added with stirring to dissolve, then potassium carbonate (18.51 mmol) and 1,3-dibromopropane (36.99 mmol) were added thereto, and the reaction was carried out at room temperature overnight. , TLC tracked the completion of the reaction. After the completion of the reaction, add 90 mL of double distilled water, extract three times with 30 mL of ethyl acetate, combine the organic phases, and then use 50 mL of saturated NH 4 Cl solution and 50 mL saturated NaCl solution were washed once, and the organic phase was washed with anhydrous NaSO 4 dry. Finally, the organic phase was concentrated by rotary evaporation at 45°C, purified and isolated by column chromatography (eluent (V / V): PE / EA=2 / 1) to obtain 4-(3-bromopropanyl)-2H -benzopyran-2-one, white solid, 86.9% yield, m.p.108-109°C; 1 H NMR (400M...
Embodiment 1-2
[0060] Example 1-2: Preparation of tert-butyl 4-(3-((2-oxo-2H-benzopyran-4-yl)oxy)propyl)piperazine-1-carboxylate tert-butyl ester
[0061]
[0062] In a 100 mL round-bottomed flask, 4-(3-bromopropanyl)-2H-benzopyran-2-one (15.89 mmol) was added and dissolved in 30 mL of acetonitrile, and N-Boc piperazine (23.84 mmol) was added thereto. ), potassium carbonate (15.89 mmol) and potassium iodide (1.59 mmol), the reaction was refluxed at 75°C for 16 h, and the completion of the reaction was tracked by TLC. After the completion of the reaction, the reaction was concentrated by rotary evaporation at 45°C, 90 mL of double distilled water was added, extracted twice with 40 mL of ethyl acetate, the organic phases were combined, and then washed twice with 50 mL of saturated NaCl solution, and the organic phase was washed with anhydrous NaSO 4 dry. Finally, it was concentrated with a rotary evaporator at 45°C, purified and isolated by column chromatography (eluent (V / V): DCM / MeOH=20 / ...
Embodiment 1-3
[0063] Example 1-3: Preparation of 4-(3-(piperazin-1-yl)propoxy)-2H-benzopyran-2-one
[0064]
[0065] tert-Butyl 4-(3-((2-oxo-2H-benzopyran-4-yl)oxy)propyl)piperazine-1-carboxylate tert-butyl ester was dissolved in 10 mL of dichloromethane, and then added to 1 mL of trifluoroacetic acid was added thereto, and the reaction was stirred at room temperature for 2 h. After the solvent was removed by rotary evaporation, 4-(3-(piperazin-1-yl)propoxy)-2H-benzopyran-2-one was obtained, and the next experiment was carried out directly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com