Carbonyl reductase mutant with improved thermal stability
A mutant and reductase technology, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as pollution and low efficiency, and achieve the effect of improving catalytic efficiency and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1. Prediction and screening of mutation sites
[0015] A) The recombinant plasmid pET-28a(+)-ChKRED20 constructed by carbonyl reductase ChKRED20 was previously constructed, the nucleotide sequence is shown in SEQ ID NO.1, and the amino acid sequence is shown in SEQ ID NO.2 (Chinese Patent, CN103497911A ).
[0016] B) By analyzing the amino acid sequence and three-dimensional structure of carbonyl reductase ChKRED20, the potential stable mutation sites were predicted and screened. The selected sites are shown in Table 1.
[0017] First, we used the amino acid sequence SEQ ID NO. 2 of the wild-type carbonyl reductase ChKRED20 as a probe to search for sequence similarity in the NCBI database, and selected homology with sequence similarity between 30-60% and 60-90%, respectively. Sequence, and then through ClusterW multiple sequence alignment to obtain the amino acids with a frequency of more than 50% at different positions, which are used as the prediction of the ...
Embodiment 2
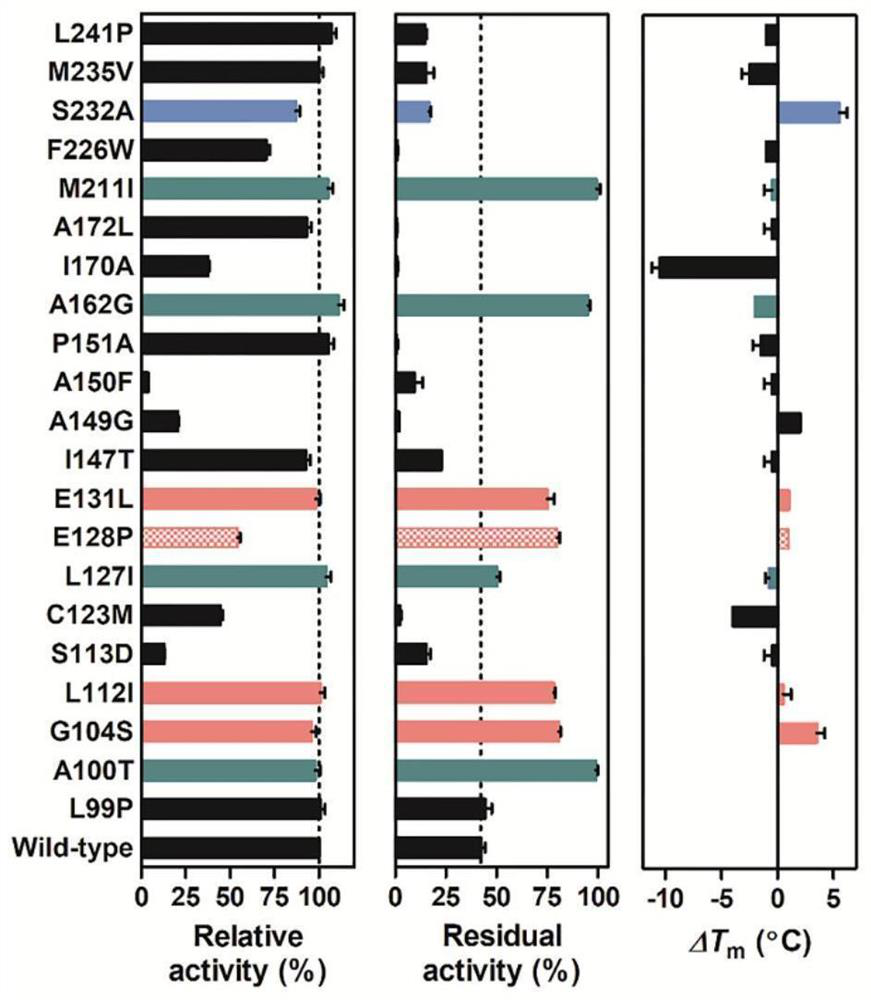
[0024] Example 2. Stability analysis of carbonyl reductase single point mutants
[0025] Thermodynamic stability test: using differential scanning fluorescence method to measure the melting temperature of enzyme protein (T m ) to measure: 10× SYPRO Orange staining solution and 20 mM mutant pure enzyme were mixed, and then 100 mM potassium phosphate buffer pH 8.0 was added to make the volume of the whole system 20 μL. In the CFX96 real-time quantitative PCR instrument, the temperature was increased from 5 °C to 95 °C at a speed of 1 °C / min, and the melting temperature of the enzyme protein was determined by observing the change of fluorescence intensity during the whole heating process (T m ).
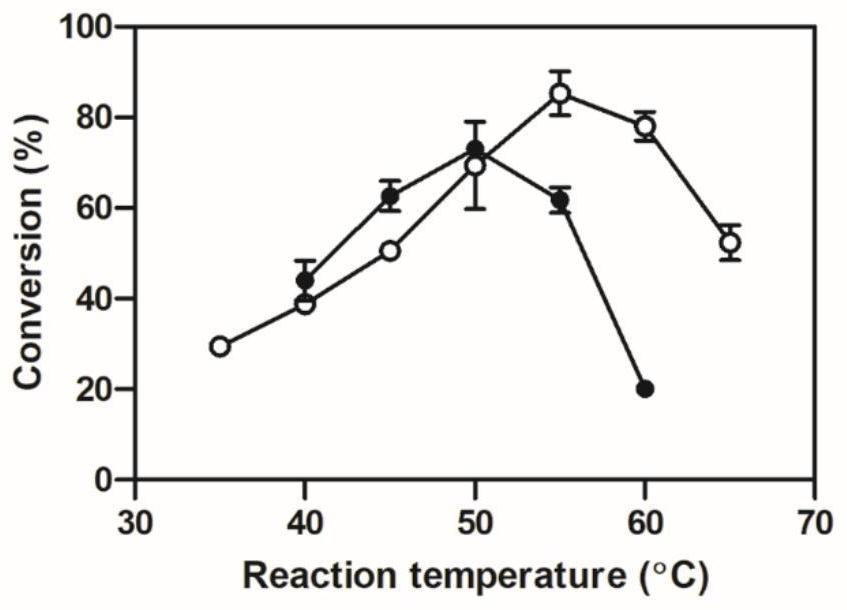
[0026] Kinetic stability test: The catalytic system was set up with 3,5-bis-trifluoromethylacetophenone as the substrate, and the catalytic activity of the enzyme to the substrate was determined. The reaction system was 1 mL, which contained 1.6 mg / mL of crude enzyme solution, 30 mM s...
Embodiment 3
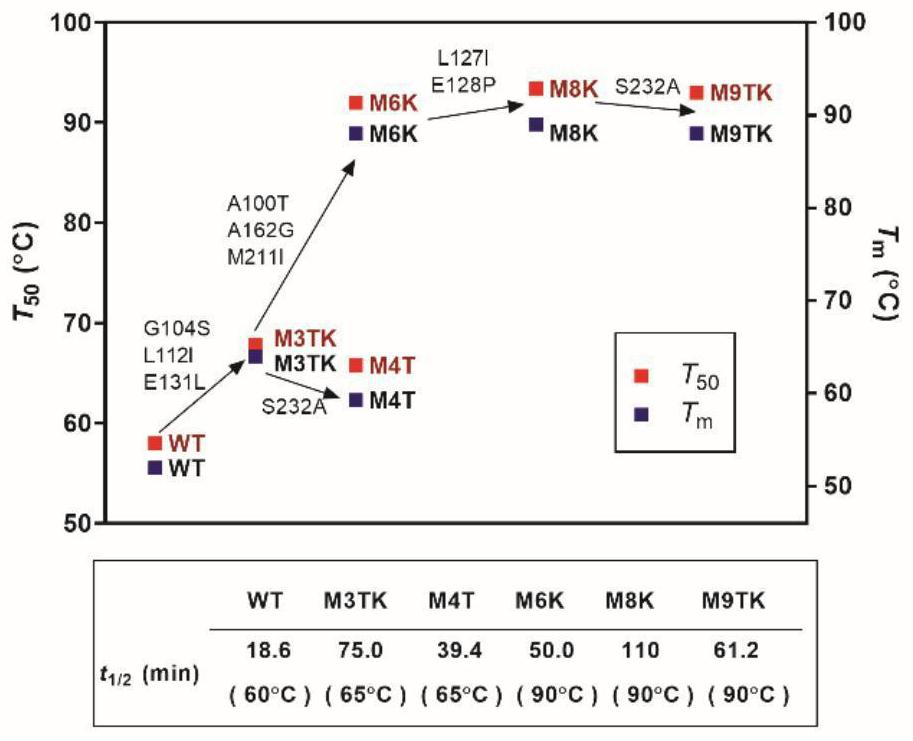
[0028] Example 3. Combination of single point mutation sites and stability analysis of combined mutants
[0029] According to the differences in the thermodynamic or kinetic stability of the single-point mutation sites, they are divided into three groups: thermodynamically stable mutants, kinetically stable mutants, and mutants with both kinetics and thermodynamics stability. Then, the mutation sites within or between groups were combined to obtain 5 combined mutants with significantly improved stability, namely M3TK, M4T, M6K, M8K, and M9TK, which were characterized as follows:
[0030] M3TK: Glycine at position 104 was mutated to serine, leucine at position 112 was mutated to isoleucine, and glutamic acid at position 131 was mutated to leucine.
[0031] M4T: Glycine at position 104 was mutated to serine, leucine at position 112 was mutated to isoleucine, glutamic acid at position 131 was mutated to leucine, and serine at position 232 was mutated to alanine.
[0032] M6K: Al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com