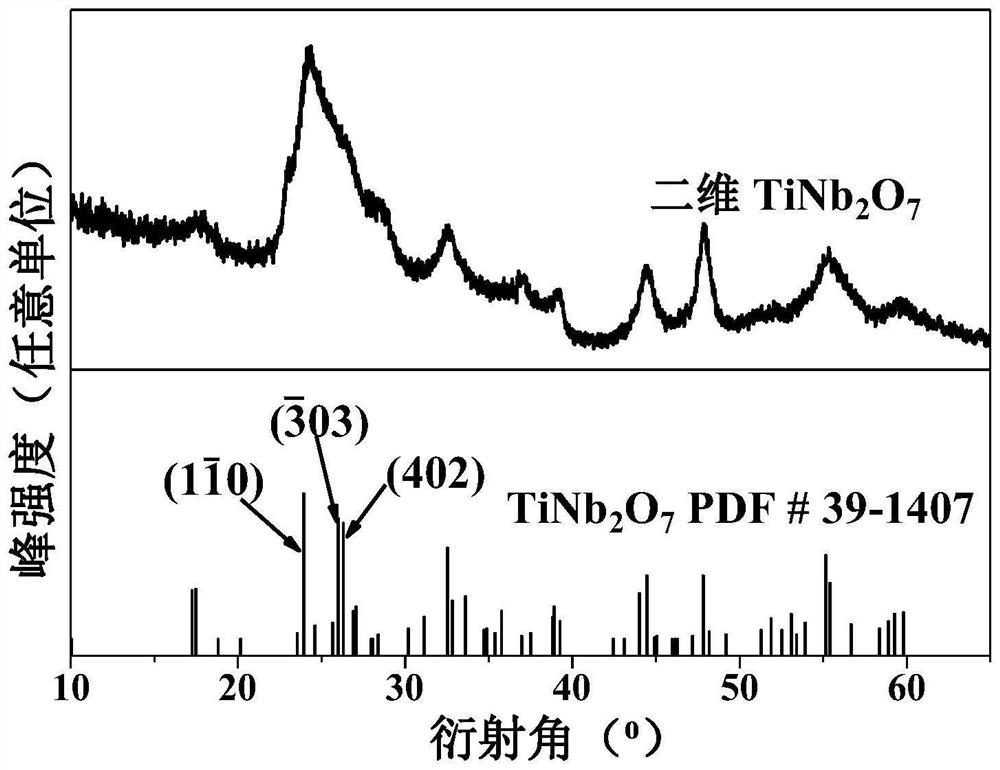
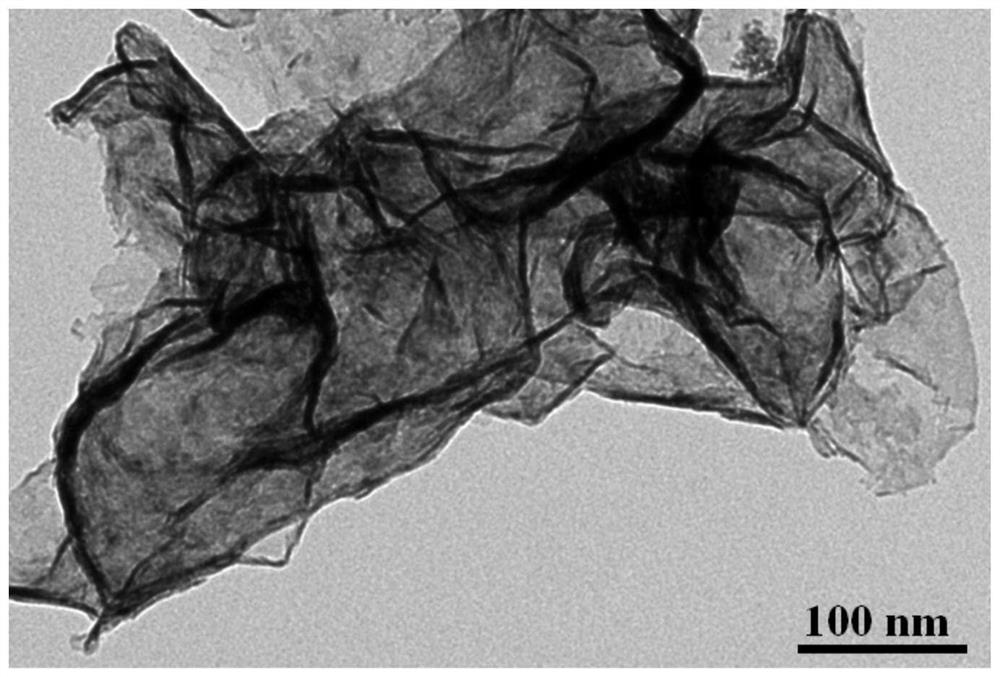
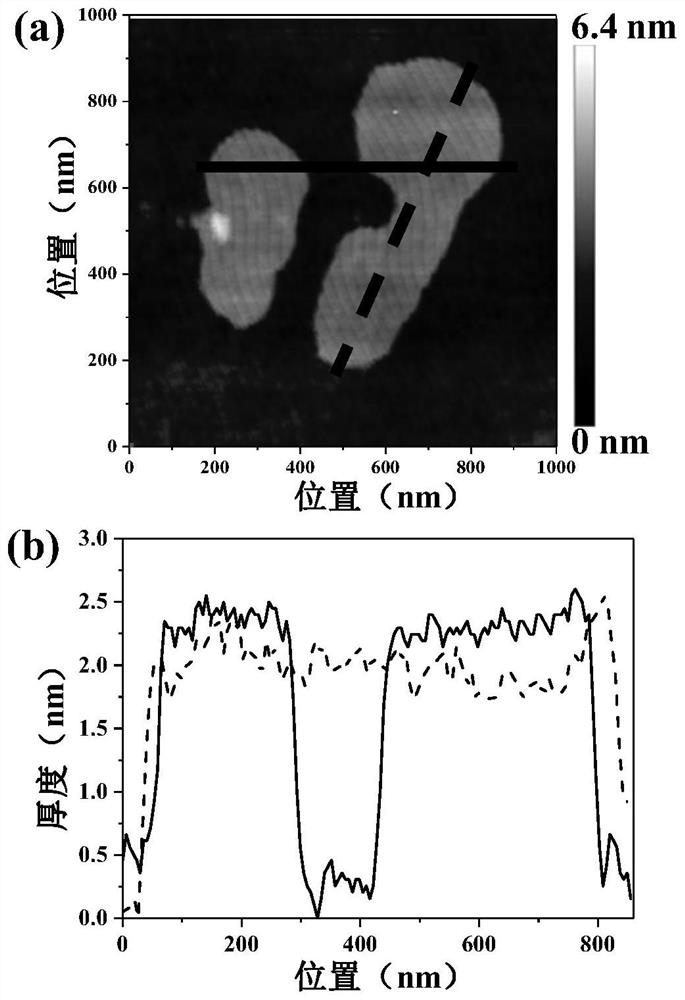
Bimetallic titanium niobium oxide, preparation method thereof and application of bimetallic titanium niobium oxide as catalyst of hydrogen storage material
A titanium-niobium oxide and hydrogen storage material technology, applied in the field of hydrogen storage materials, can solve the problems of low catalytic efficiency, loss of effective hydrogen storage capacity, etc., and achieve high catalytic efficiency, improved hydrogen absorption and desorption kinetic performance and cycle performance, The effect of large contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of lamellar titanium niobium oxide:
[0056] (1) 0.78 g of titanium n-butoxide and 1.30 g of niobium ethoxide were added to 1.33 g of concentrated hydrochloric acid with a concentration of 38% under stirring to form mixed solution A.
[0057] (2) Add 0.36 g of surfactant polyoxyethylene-polypropylene ether block copolymer (F127) into 6.7 mL of absolute ethanol, and stir until F127 is completely dissolved in absolute ethanol to form mixed solution B.
[0058] (3) Add the above mixed solution B into the above mixed solution A, and continue to stir to form a mixed solution C. Then 35.6mL of ethylene glycol was added to 4.4mL of mixed solution C under stirring, the mixed solution was transferred to an 80mL reaction kettle, heated to 150°C in an oven and kept for 20 hours, and cooled to room temperature with the furnace, solid-liquid A precipitate was isolated.
[0059] (4) The obtained precipitate was centrifugally washed with absolute ethanol to remove surfac...
Embodiment 2
[0079] The preparation of lamellar titanium niobium oxide is the same as that in Example 1.
[0080] Using the layered titanium niobium oxide as catalyst, added to MgH 2 In the hydrogen storage material, the addition amount is 1 wt %, and the ball milling is mixed, and the ball milling process is the same as that of Example 1.
[0081] Using the volume method at an initial vacuum of 10 –3 Under the condition of Torr, the kinetic performance of hydrogen release with temperature was tested, and the obtained hydrogen release curve with temperature was as follows: Figure 8 As shown, the initial hydrogen release temperature and the end temperature of hydrogen release are 206 °C and 310 °C, respectively, and the amount of hydrogen released when heated to 300 °C is as high as 7.2 wt%.
Embodiment 3
[0083] The preparation of lamellar titanium niobium oxide is the same as that in Example 1.
[0084] Using the layered titanium niobium oxide as catalyst, added to MgH 2 In the hydrogen storage material, the addition amount is 5 wt %. Mixing by ball milling, the ball milling process is the same as that in Example 1.
[0085] Using the volume method at an initial vacuum of 10 –3 The kinetic properties of hydrogen release with temperature of the test material were tested, and the obtained hydrogen release curve with temperature was as follows: Figure 9 As shown, the initial hydrogen release temperature and the end temperature of hydrogen release are 175°C and 295°C, respectively, and the amount of hydrogen released when heated to 300°C is as high as 6.8 wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com