Electron-deficient heterocyclic core small-molecule electron donor material as well as preparation and application thereof
An electron donor material and core small molecule technology, applied in the field of solar cells, can solve problems such as scarcity of optimizeable sites and modifiable units, single structure of oligothiophene, and few optimization strategies, and achieve excellent photoelectric conversion efficiency and synthetic route Less, avoid complex effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
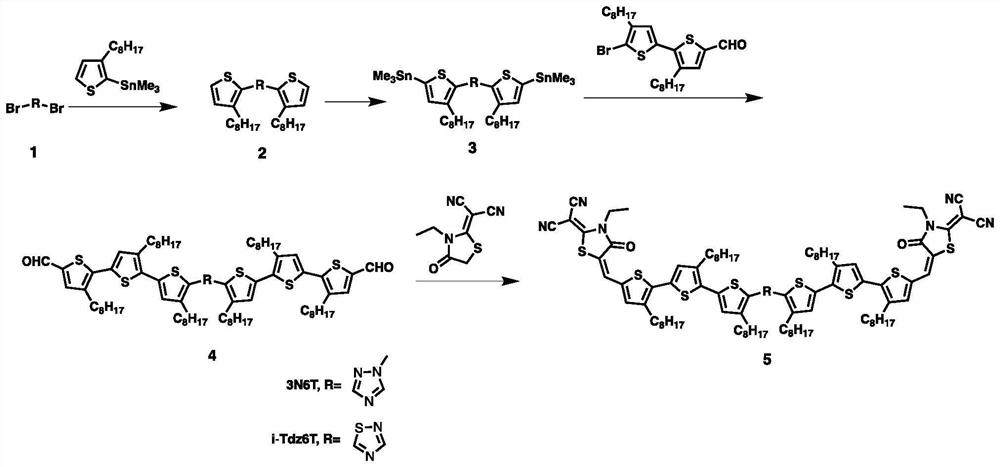
[0082] like figure 1 As shown, the preparation process of the electron-deficient heterocyclic core small molecule electron donor material in this embodiment is as follows:
[0083] 1. Synthesis of compound 2
[0084] Compound 1 (1.0 equiv. purchased from Anaiji) and octylthiophene tin reagent (2.4 equiv. purchased from Gea) were dissolved in 50 ml of anhydrous toluene, and then Pd(PPh3)4 (tetrakistriphenylphosphine palladium) 150 mg. The reaction was stirred at 130°C for 48 hours. Then the solvent was removed under reduced pressure, separated by silica gel column chromatography, the eluent was chloroform / petroleum ether (by volume, chloroform:petroleum ether=1:6), the product (compound 2) was a pale yellow oil (the yield was the theoretical yield) 65%).
[0085] Characterization data for compound 2: 1H NMR (400 MHz, Acetone, δppm): 7.72 (d, J=5.2 Hz, 1H), 7.39 (d, J=4.8 Hz, 1H), 7.20 (d, J=5.2 Hz, 1H) ),7.04(d,J=5.2Hz,1H),3.97(s,3H),3.18(t,J=7.6Hz,2H),2.85-2.81(m,3H),1.73...
Embodiment 2
[0095] like figure 1 As shown, the preparation process of the electron-deficient heterocyclic core small molecule electron donor material in this embodiment is as follows:
[0096] 1. Synthesis of compound 2
[0097] Dihalogenated isothiadiazoles (1.0 equiv. purchased from Anaiji) and octylthiophene tin reagent (2.4 equiv. purchased from Gea) were dissolved in 50 ml of anhydrous toluene, and then Pd(PPh3)4 (tetratriphenylene) was added phosphine palladium) 150 mg. The reaction was stirred at 130°C for 48 hours. Then the solvent was removed under reduced pressure, separated by silica gel column chromatography, the eluent was chloroform / petroleum ether (by volume, chloroform:petroleum ether=1:6), and the product (compound 2) was a pale yellow oil (the yield was the theoretical yield 40%).
[0098] Characterization data of compound 2: 1H NMR (400MHz, CDCl3, δppm): 7.45 (d, J=4.8Hz, 1H), 7.32 (d, J=5.2Hz, 1H), 7.03 (d, J=5.2Hz, 1H) ),6.99(d,J=4.8Hz,1H),3.23(t,J=7.6Hz,2H),2.91...
Embodiment 3
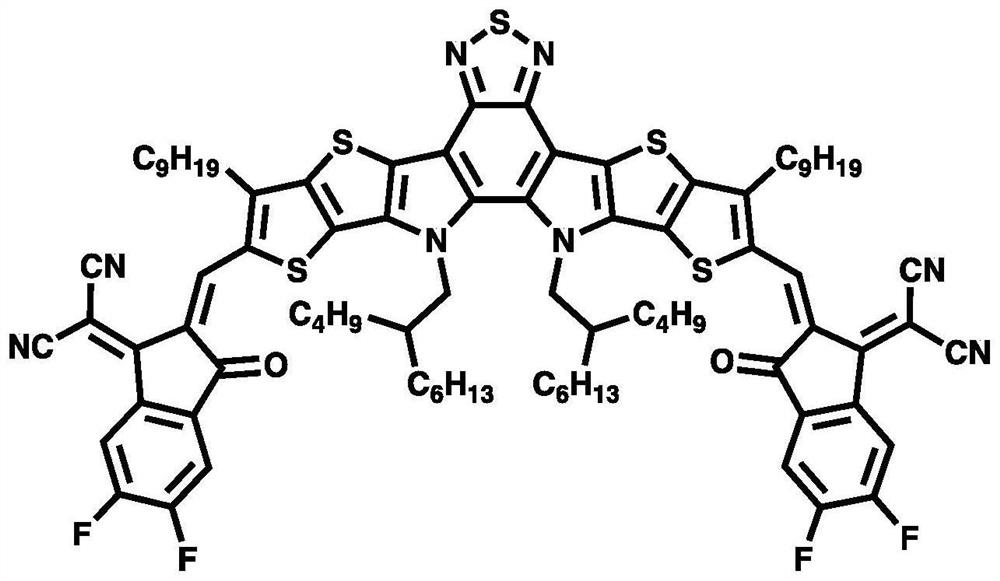
[0108] The electron-deficient heterocyclic core small molecule electron donor material (ie figure 1 3N6T) as shown in the preparation of an all-small-molecule organic solar cell, the specific preparation process is as follows:
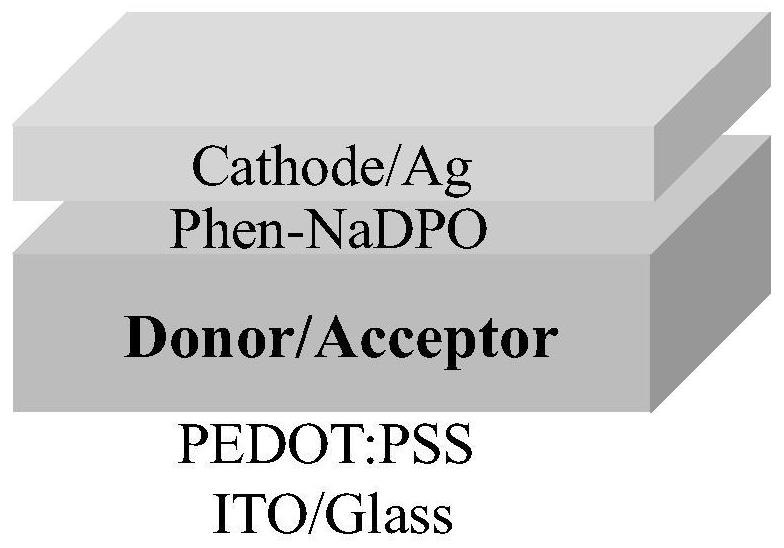
[0109] The substrate composed of transparent glass and transparent conductive electrode ITO was ultrasonically cleaned with cleaning solution, deionized water, acetone and isopropanol respectively, and dried with nitrogen after cleaning; the substrate was placed in an ozone cleaning machine for 15min. , the hole transport layer material PEDOT:PSS was spin-coated in air (4000rpm, 20s, film thickness 30nm), followed by thermal annealing in air (120°C, 10min), and then the samples were transferred into a nitrogen-filled glove box , the active layer (3N6T:BTP-eC9-4F=2:1, 40mg / ml, active layer thickness: ≈ 200nm) was prepared by spin coating on the PEDOT:PSS hole transport layer, and the obtained active layer thin film Solvent annealing treatment (CF, 30 s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com