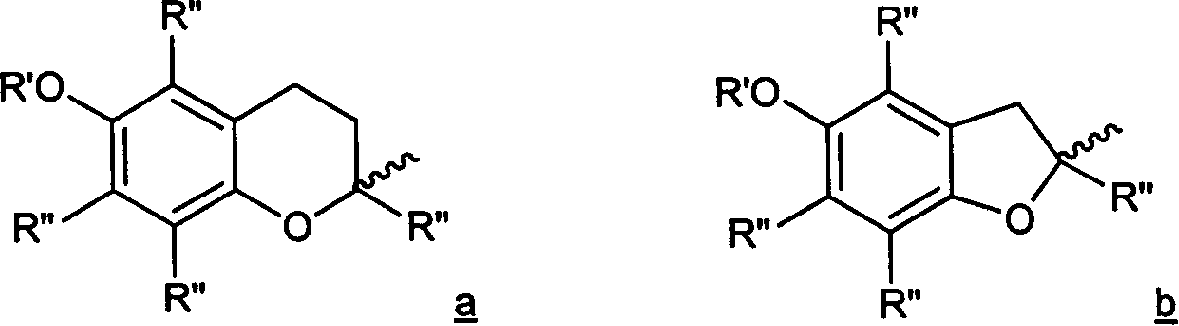
Inhibitor used as antioxygen and 5-lipoxygenase, and anti-inflammatory non-steroid carboxylic ester and amide of non-steroid anti-inflammatory agent
A non-steroidal, carboxylic acid technology, applied in the direction of anti-toxic agents, anti-inflammatory agents, medical preparations of non-active ingredients, etc., can solve side effects and other problems, and achieve the effect of increasing lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Synthesis of N-(2-(3,4-dihydroxyphenyl)-2-hydroxyethyl)-N-methyl 2-(6 -Methoxy-2-naphthyl)propionamide
[0134]Epinephrine (Aldrich, 3.18 grams [g], 17.3 mmol [mmol]), 1-hydroxybenzotriazole hydrate (Aldrich, 1.76 g, 12.9 mmol) and 1-(3-dimethylaminopropyl )-3-Ethylcarbodiimide HCl (Aldrich, 2.49 g, 12.9 mmol) was added to acetonitrile (200 milliliters [ml]). After stirring for 10 minutes, a solution of 6-methoxy-α-methyl-2-naphthaleneacetic acid (Aldrich, 2.0 g, 8.66 mmol) in 50 ml of acetonitrile was added dropwise. After stirring for 16 hours, the reaction mixture was concentrated in vacuo (reduced pressure) and the residue was partitioned between water (100ml) and dichloromethane (100ml). The layers were separated and the aqueous layer was extracted with dichloromethane (2 x 50ml) and ethyl acetate (50ml). The combined organic phases were treated with methanol until a clear solution formed. The solution was dried (magnesium sulfate) and concentrated in vacuo....
Embodiment 2
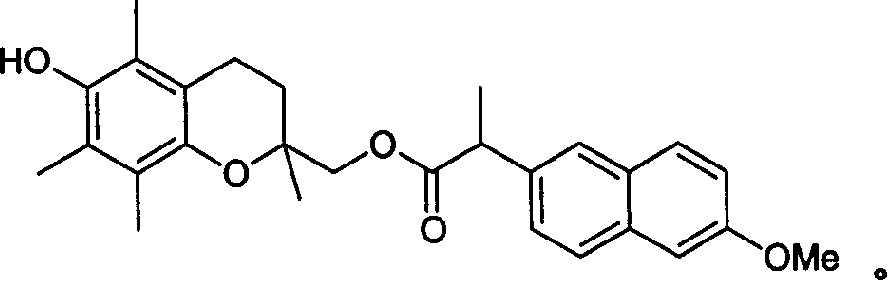
[0141] Synthesis of 2-(6-hydroxy-2,5,7,8-tetrafluoroethylene)propionate 2-(6-methoxy-2-naphthyl) Methyl-3,4-dihydro-2H-benzo[1,2-b]pyran-2-yl)methyl ester
[0142] Under nitrogen atmosphere, 6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzo[1,2-b]pyran-2-yl)methanol (2.00g, 8.46mmol), 6-Methoxy-α-methyl-2-naphthaleneacetic acid (2.14g, 9.31mmol), dimethylaminopyridine (Aldrich, 1.24g, 10.00mmol) and 1-(3-dimethylaminopropyl) - A solution of 3-ethylcarbodiimide hydrochloride (1.71 g, 8.89 mmol) in tetrahydrofuran (40 ml) was stirred at room temperature for 72 hours. The mixture was then diluted with ethyl acetate (200ml) and washed first with 0.5N hydrochloric acid (2x250ml) followed by water (2x250ml), then dried (sodium sulfate) and concentrated in vacuo. The residue was flash chromatographed (silica gel, 100-50:0-50, V:V, hexane:ethyl acetate) and the appropriate fractions were concentrated to give an oil. Recrystallization from ethyl acetate-hexane gave 2.21 g (58.3% yield) of...
Embodiment 3
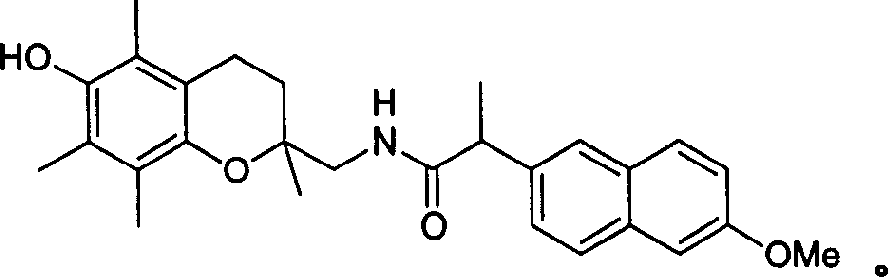
[0149] Synthesis of N-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzene And[1,2-b]pyran-2-yl)methyl)]-2-(6-methoxy-2-naphthyl)propionamide
[0150] First synthesize the intermediate (6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-1-benzo[1,2-b]pyran-2-yl)methanamine :
[0151] A 1M solution of lithium aluminum hydride in diethyl ether (Aldrich, 32.4 mL, 32.43 mmol) was slowly added dropwise to the stirred (2-cyano-6-hydroxy-2,5,7,8-tetramethyl -3,4-dihydro-2H-1-benzo[1,2-b]pyran in tetrahydrofuran (50ml) cold (4-6 ° C) solution. After 2 hours, under stirring, slowly add 10 % hydrated tetrahydrofuran (30 mL), 15% sodium hydroxide (10 mL) and water (20 mL), quenched the reaction mixture. The resulting suspension was filtered through celite, and the celite plate was washed with diethyl ether (400 mL). The organic layer was dried (Na 2 SO 4 ), and concentrated in vacuo to obtain a residue. To a solution of the residue in ether (100 mL) was then added 1M hydrogen chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com