Water-soluble medicine particle-type slow-release composition, preparation and its preparing method
A technology for water-soluble drugs and compositions, applied in the directions of drug combinations, pharmaceutical formulations, pill delivery, etc., can solve the problems of increasing the rate of drug absorption, unable to adjust the dose, and increasing the side effects of drugs, so as to improve the stability and process production. The process is simple and easy to operate, and the effect of strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of albuterol sulfate controlled-release microspheres
[0045] prescription:
[0046] Salbutamol Sulfate 5.0g
[0047] Amberlite-IRP88 5.0g
[0048] EUDRAGIT® RSPO 37.5g
[0049] EUDRAGIT® L100 6.25g
[0050] Triethyl citrate 5.0g
[0051] Magnesium Stearate 2.5g
[0052] Absolute ethanol 200ml
[0053] Liquid paraffin 600ml
[0054] Preparation:
[0055] (1) preparation of medicine resin: the salbutamol sulfate water is made into the solution that concentration is 50~150mg / ml, drips in the glass exchange column that the ion exchange resin Amberlite-IRP88 of recipe quantity is housed, adopts dynamic adsorption method to carry out medicine, After the exchange is complete, rinse with deionized water until the drug concentration cannot be detected, dry and set aside;
[0056] (2) Preparation of microspheres: Dissolve acrylic resin EUDRAGIT® RSPO, EUDRAGIT® L100 and triethyl citrate in absolute ethanol, add medicinal resin and magnesium stearate to make ...
Embodiment 2
[0080] Preparation of albuterol sulfate sustained-release microspheres: (preparation of sustained-release granules and suspensions)
[0081] Preparation of microspheres:
[0082] prescription:
[0083] Salbutamol Sulfate 5.0g
[0084] Amberlite-IRP88 5.0g
[0085] EUDRAGIT RSPO 60.0g
[0086] EUDRAGIT L100 10.0g
[0087] Dimethyl phthalate 6.0g
[0088] Magnesium Stearate 3.0g
[0089] Absolute ethanol 200ml
[0090] Simethicone 600ml
[0091] The preparation method is the same as in Example 1.
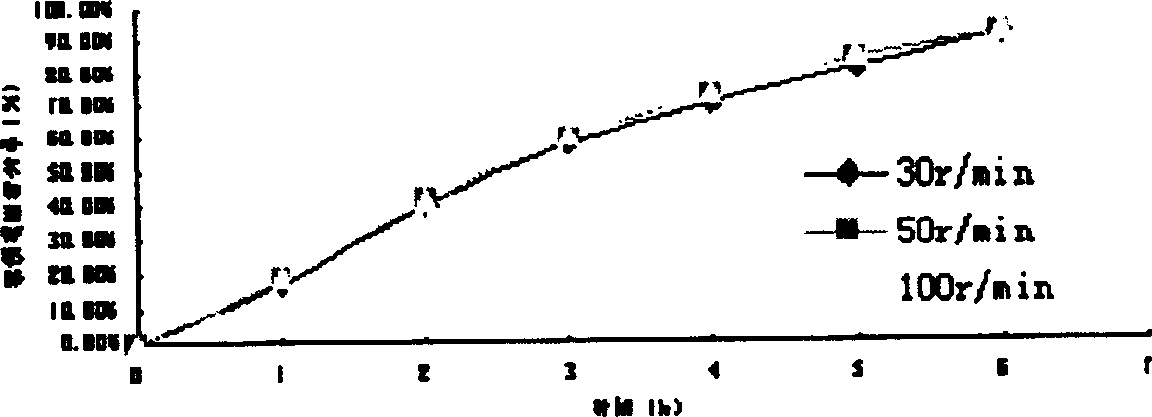
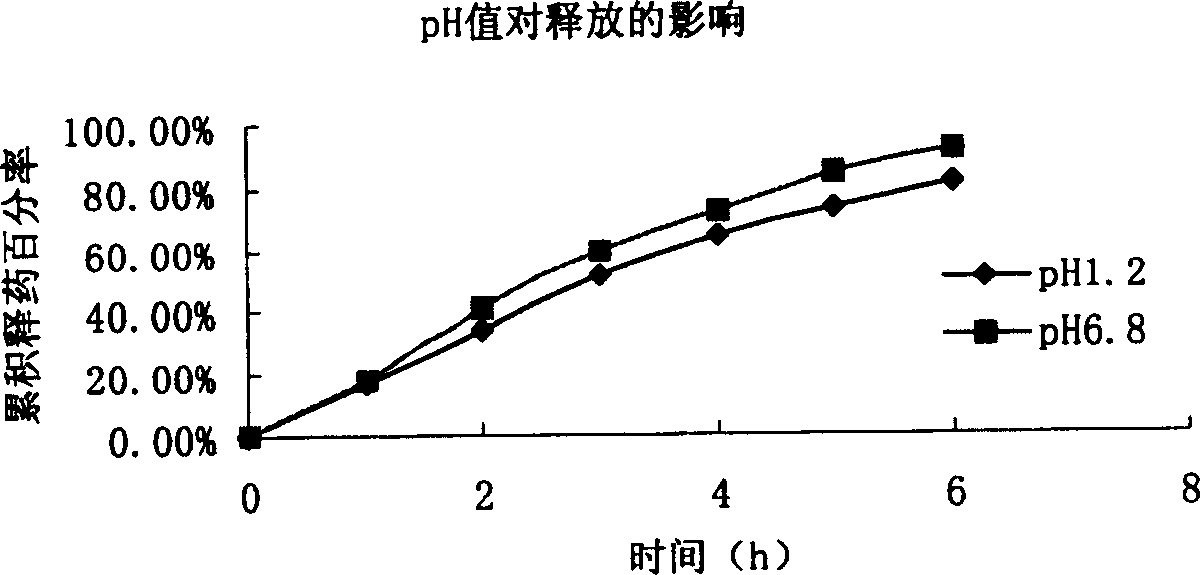
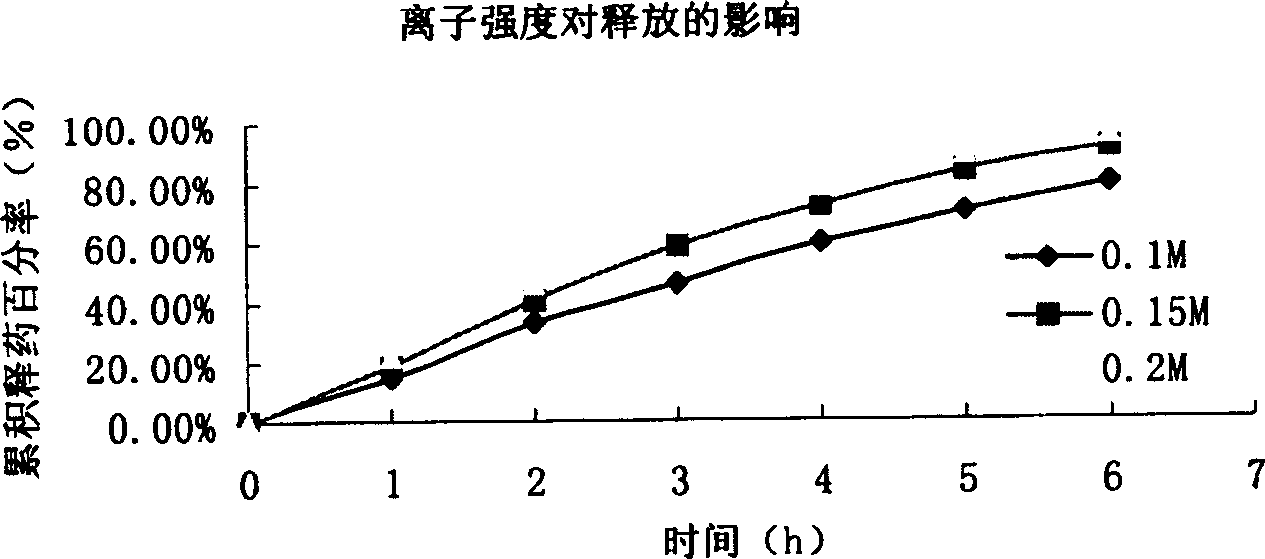
[0092] In vitro release of microspheres: the effects of rotational speed, pH value, salt ionic strength, and particle size on the release of microspheres are the same as in Example 1. Depending on the particle size, it can provide sustained release for 3-8 hours. The sphericity, particle size and distribution measurements of the microspheres were similar to Example 1.
[0093] Sustained-release granules are prepared by conventional methods in the art:
[0094] Sustai...
Embodiment 3
[0098] prescription:
[0099] Salbutamol Sulfate 5.0g
[0100] Amberlite IRP88 5.0g
[0101] EUDRAGIT RSPO 36g
[0102] EUDRAGIT L100 12g
[0103] Triethyl citrate 2.5g
[0104] Magnesium Stearate 1.0g
[0105] Dichloromethane 200ml
[0106] Corn Oil 600ml
[0107] In vitro release of microspheres: the effects of rotational speed, pH value, salt ionic strength, and particle size on the release of microspheres are the same as in Example 1. Depending on the particle size, it can provide sustained release for 4-10 hours. The sphericity, particle size and distribution determination of the microspheres are similar to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com