Liposome anti-fungus medication sprayer formulation
A technology of antifungal drugs and sprays, which is applied in the field of medicine, can solve problems such as inability to eradicate patients, and achieve the effects of enhancing drug action time, avoiding irritation, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Contain 0.1% mole miconazole nitrate in the drug liposome product.
[0053] name
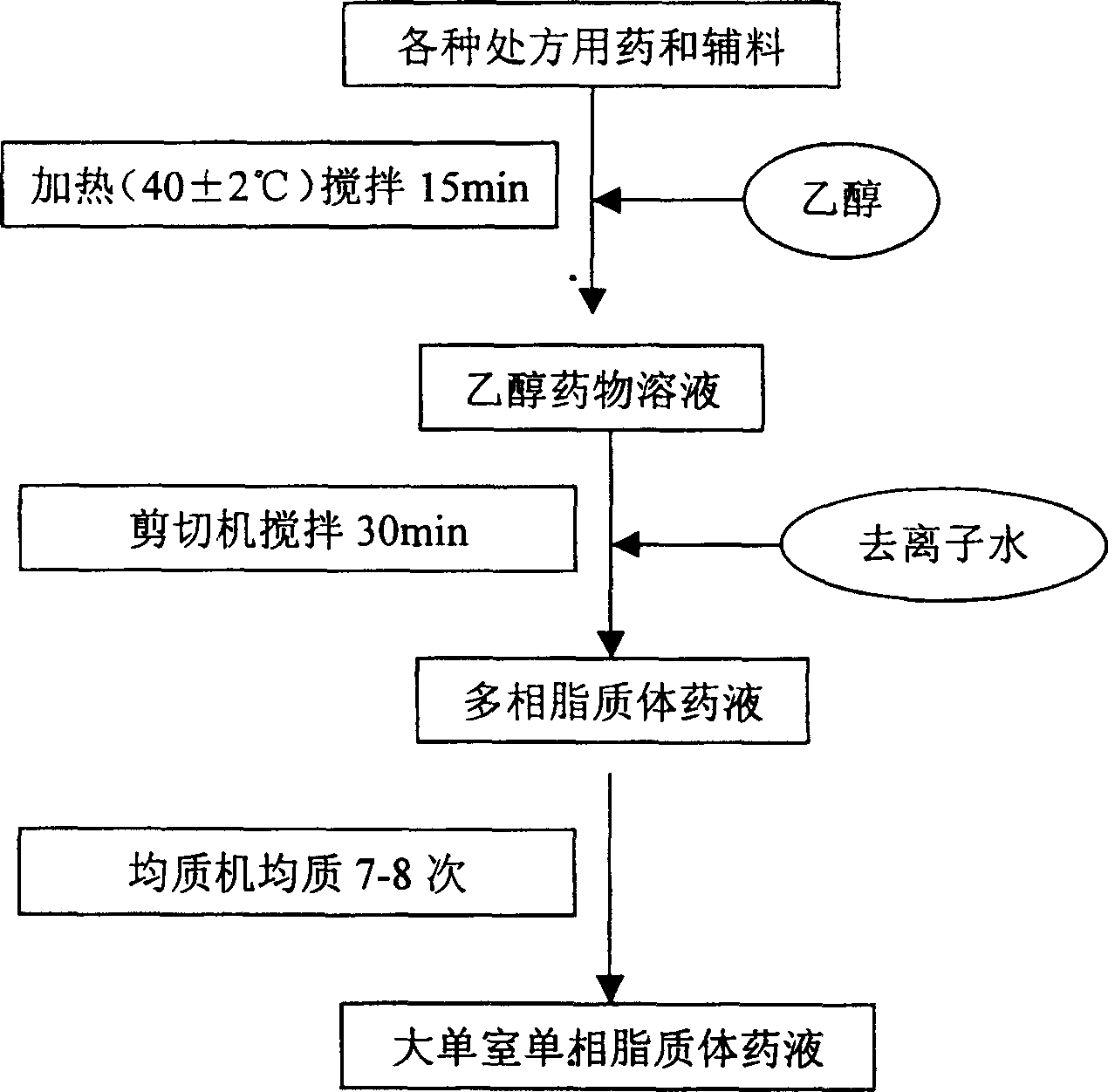
[0054] Add the prescribed amount of lecithin, cholesterol, oleic acid, miconazole nitrate, azone, vitamin E and peppermint oil into 20ml of ethanol, heat to 45°C and stir to dissolve completely. Then slowly add the ethanol drug solution into 180ml of pure water stirred at a high speed (3000r / min) by a shearing machine, and continue to stir for 30min under nitrogen protection to obtain the multiphase liposome drug solution. The multiphase liposome drug solution was homogenized 7 times by a high-pressure homogenizer, and the pressure was 100 MPa. During the homogenization process, the temperature should not exceed 60°C to obtain a large single-chamber single-phase liposome drug solution with a particle size of 250nm ± 40nm. The final product after dilution is a uniform milky solution without precipitation and stratification. Store the product at 2°C-15°C and dark ...
Embodiment 2
[0055] Embodiment 2: Contain 15% mole miconazole nitrate in the drug liposome product.
[0056] name
molecular weight
Mole (%)
Feed amount (g)
760
74.0
18.7
386.66
7.5
1.3
479.15
15.0
2.4
282
3.0
0.3
281
0.1
0.01
Vitamin E
472
0.2
0.03
peppermint oil
0.2
0.3
[0057] Add 40ml of ethanol to the prescribed amount of lecithin, cholesterol, oleic acid, miconazole nitrate, azone, vitamin E and peppermint oil, heat and stir until completely dissolved. Slowly add the ethanol drug solution into 160ml of pure water stirred at high speed (3000r / min) by a shearing machine, and continue to stir for 35min under nitrogen protection to obtain the multiphase liposome drug solution. The multiphase liposome drug solution was homogenized 6 tim...
Embodiment 3
[0059] Embodiment 3: Contain 7% mole miconazole nitrate in the drug liposome product.
[0060] name
molecular weight
Mole (%)
Feed amount (g)
760
82.0
19.11
cholesterol
386.66
7.5
0.89
miconazole nitrate
479.15
7.0
0.10
282
3.0
0.3
281
0.1
0.01
Vitamin E
472
0.2
0.03
peppermint oil
0.2
0.3
[0061] Add the prescribed amount of lecithin, cholesterol, oleic acid, miconazole nitrate, azone, vitamin E and peppermint oil into 30ml of ethanol, heat to 45°C and stir to dissolve completely. Slowly add the ethanol drug solution into 170ml of pure water stirred at a high speed (3000r / min) by a shearing machine, and continue stirring for 30min under nitrogen protection to obtain the multiphase liposome drug solution. The multiphase liposome drug solution was homogen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com