Nobonene-ester based addition polymer and method for preparing the same
A norbornene and polymer technology, applied in the field of norbornene-based addition polymers, can solve the problems of polymerization yield and molecular weight reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0130] All procedures for handling air- and light-sensitive compounds were performed by standard Schlenk techniques or using drying boxes. Nuclear magnetic resonance (NMR) spectra were obtained using a Bruker 600 spectrometer and a Bruker 300 spectrometer. 1 H NMR at 600MHz or 300MHz, 13 CNMR was measured at 150MHz and 75MHz. For definite analysis of NMR signals, 2-dimensional assays such as COSY and HMBC were performed. The molecular weight and molecular weight distribution of the polymers were determined by GPC (gel permeation chromatography) using polystyrene samples as standards. Thermal analysis, such as TGA and DSC, was performed using a TA instrument (TGA2050; heating rate = 10 K / min).
[0131] Toluene was purified by distillation in potassium / benzophenone, CH 2 Cl 2 by CaH 2 purified by distillation.
Embodiment 1
[0136] Example 1: Preparation of exo-rich norbornene carboxylate methyl ester (180°C, 6 hours, reaction coefficient = 84,993)
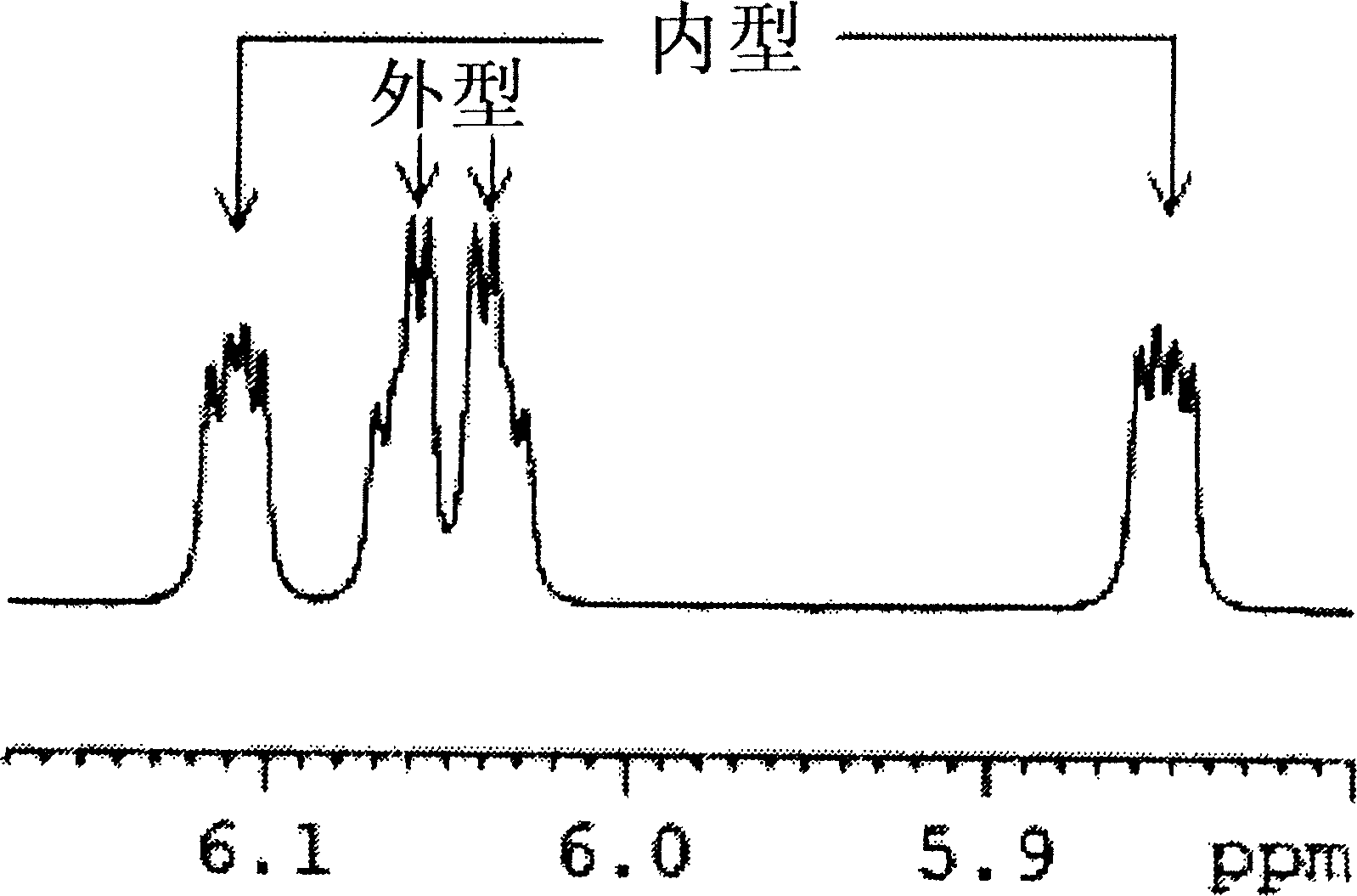
[0137] DCPD (Aldrich, 256.5 mL, 1.9 mol), methacrylate (Aldrich, 405 mL, 4.5 mol) and hydroquinone (3.2 g, 0.03 mol) were placed in a 2 L autoclave. After heating to 180°C, the autoclave was stirred at 300 rpm to allow the reaction to proceed for 6 hours. After the reaction was completed, the reaction mixture was cooled and then transferred to a distillation apparatus. The reaction mixture was distilled at 50°C under 1 Torr using a vacuum pump to obtain the product (yield: 86%). The molar ratio (mol%) of exo-isomer to endo-isomer of the product was 52:48.
[0138] 1 H-NMR (600MHz, CDCl 3 ), endotype: δ6.17(dd, 1H), 5.91(dd, 1H), 3.60(s, 3H), 3.17(b, 1H), 2.91(m, 1H), 2.88(b, 1H), 1.90 (m, 1H), 1.42 (m, 2H), 1.28 (m, 1H); Appearance: δ6.09 (m, 2H), 3.67 (s, 3H), 3.01 (b, 1H), 2.88 (b, 1H), 2.20 (m, 1H), 1.88 (m, 1H), 1.51 (d, 1H), 1.34 (m, 2H). ...
Embodiment 2
[0140] Example 2: Preparation of exo-rich norbornene carboxylate butyl ester (190°C, 5 hours, reaction coefficient = 89,424)
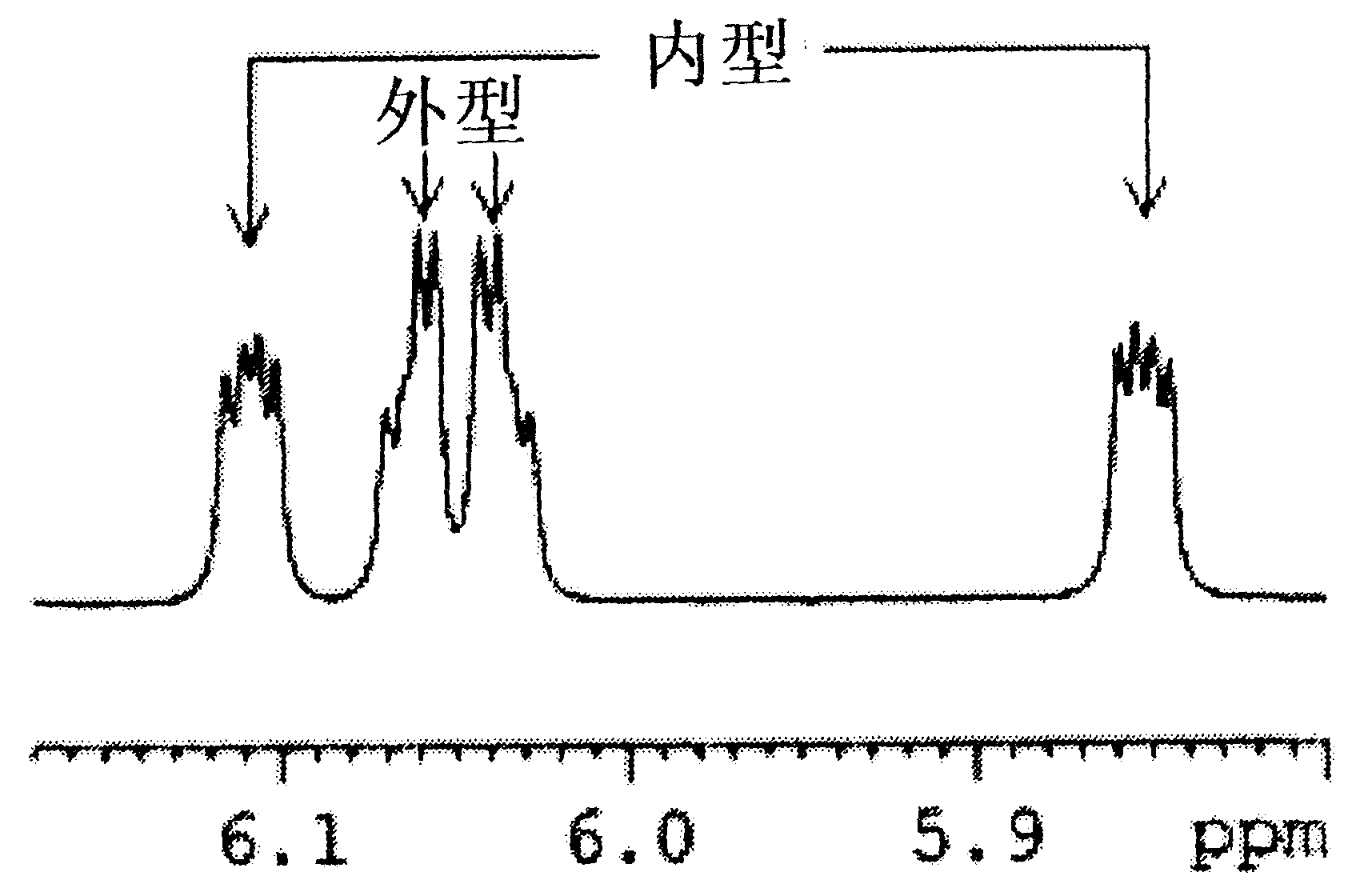
[0141] DCPD (Aldrich, 180 mL, 1.34 mol), butyl acrylate (Junsei, 500 mL, 3.49 mol) and hydroquinone (2.7 g, 0.025 mol) were placed in a 2 L autoclave. After heating to 190°C, the autoclave was stirred at 300 rpm to allow the reaction to proceed for 5 hours. After the reaction was completed, the reaction mixture was cooled and then transferred to a distillation apparatus. The reaction mixture was distilled at 80°C under 1 Torr using a vacuum pump to obtain the product (yield: 78%). The molar ratio (mol%) of exo-isomer to endo-isomer of the product was 56.2:43.8.
[0142] 1 H-NMR (600MHz, CDCl 3 ), endotype: δ6.17(dd, 1H), 5.86(dd, 1H), 3.97(t, 2H), 3.15(b, 1H), 2.88(m, 1H), 2.85(b, 1H), 1.86 (m, 1H), 1.57 (m, 2H), 1.35 (m, 4H), 1.21 (m, 1H), 0.89 (t, 3H); Appearance: δ6.09 (m, 2H), 4.05 (t, 2H), 2.98(b, 1H), 2.86(b, 1H), 2.20(m, 1H), 1.88(m, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com