Production and use of high expression, high purity, high activity gene recombinant human lysozyme
A technology of human lysozyme and genetic recombination, which is applied in genetic engineering, plant genetic improvement, recombinant DNA technology, etc., to achieve high purity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1: the production method of the human lysozyme expressed by genetic engineering;
[0011] Materials: The expression system of genetically engineered human lysozyme is characterized by using methanolotrophic yeast as the host strain "Pichia genus strain". In the phenotype of methanol utilization, both fast methanol utilization (mut+) and slow methanol utilization (muts or mut-) were used. The smd1165(his4.prb1), smd1163(his.pep4.prb1), Gs115 and km71 strains of Pichia pastoris were mainly used. The plasmid of the genetically engineered human lysozyme of the present invention is mainly integrated into the chromosome of the above bacterial strain by using the plasmid of the pPIC9k series.
[0012] Steps: 1. Shake flask seed preparation: based on the preparation of 200 milliliters of culture medium, use H 3 PO 4 (phosphoric acid) 6 ml, MgSO 4 (magnesium sulfate) 3 grams, K 2 SO 4 (potassium sulfate) 4 grams, KOH (potassium hydroxide) 1 gram, CaSO 4 2H 2 O...
Embodiment 2
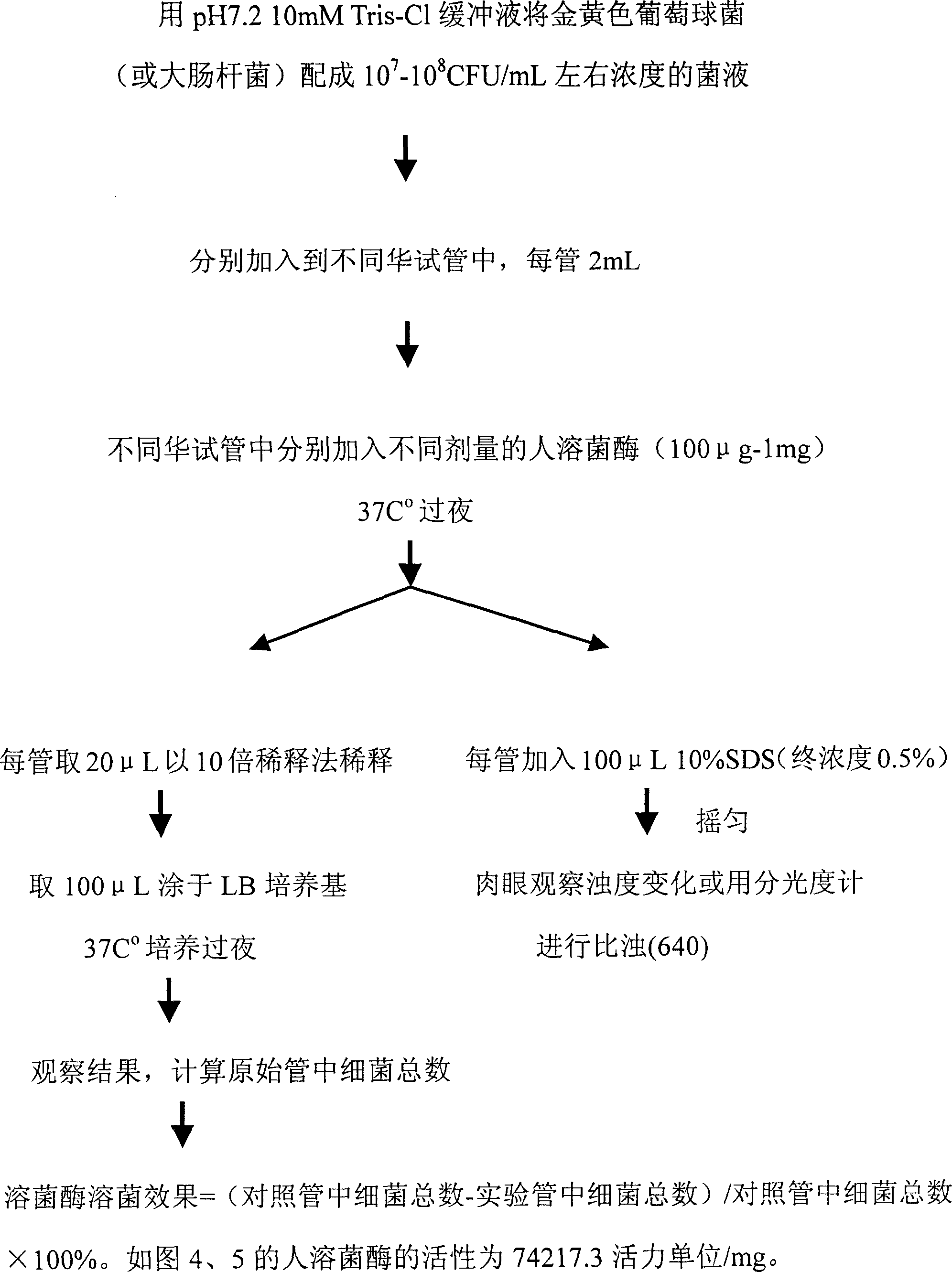
[0014] Embodiment 2: the identification of human lysozyme bacteriolytic activity (test tube method)
[0015] Material
[0016] 1. Buffer pH7.210mMTris-Cl buffer
[0017] 2. Strains Staphylococcus aureus, Escherichia coli
[0018] 3. 10% SDS
[0019] 4. LB medium
[0020] method:
[0021]
Embodiment 3
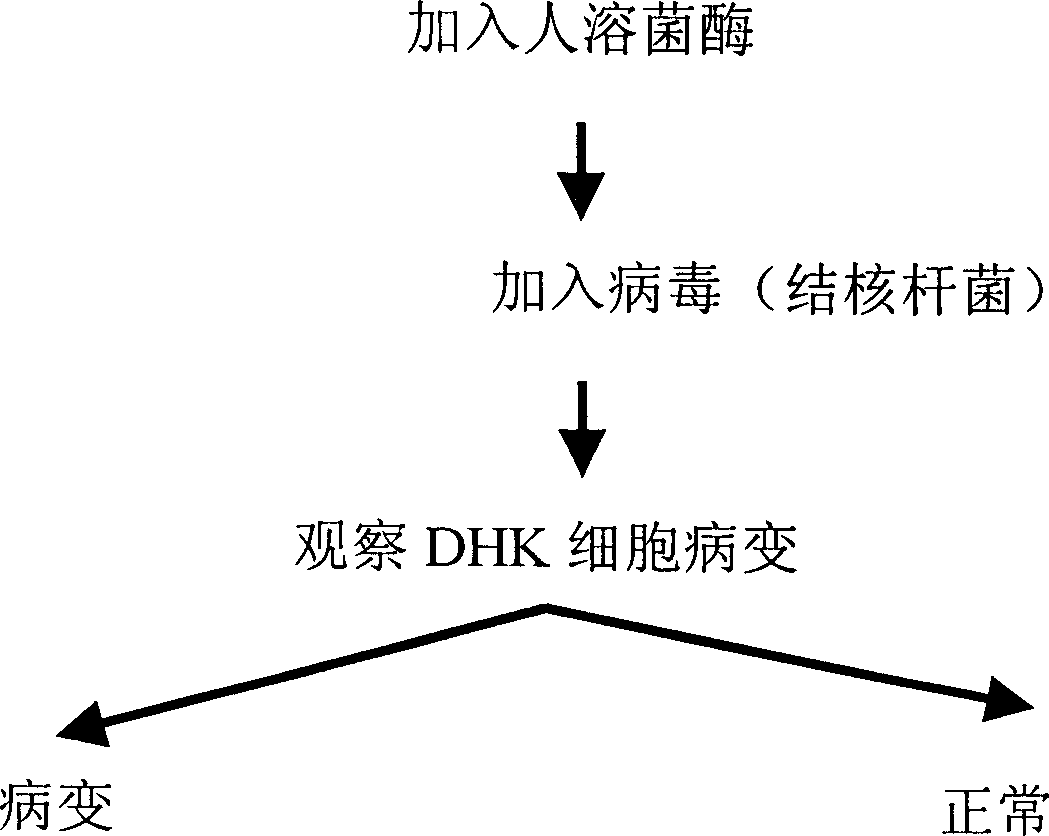
[0022] Example 3: The bactericidal effect of human lysozyme on drug-resistant bacteria, as shown in Figures 6 and 7, with Bacillus abalone and Staphylococcus aureus as examples.
[0023] Drug-resistant strains: 30 strains of different drug-resistant bacteria came from the Laboratory Department of Xi'an Xijing Hospital Method:
[0024] Prepare 1% agarose with pH7.2 10mM Tris-Cl buffer
[0025] After autoclaving, cool to 40 °C and add bacteria to 10 6 dump flat
[0026] ↓
[0027] Punch holes in the plate with a hole punch
[0028] ↓
[0029] Add different concentrations of lysozyme solution (0-100mg / ml) to the wells
[0030] ↓
[0031] Incubate in a 37°C incubator for 4-6 hours
[0032] ↓
[0033] After LB nutrient agar is autoclaved, cool to 40°C and cover on the above agarose plate
[0034] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com