Method for extracting compound of phenolic acid from leaves of woodbind
A technology for honeysuckle leaves and plant leaves, applied in the field of chlorogenic acid and isochlorogenic acid, which can solve problems affecting the extraction and separation of chlorogenic acid and isochlorogenic acid, and achieve low cost, no harmful solvents, and high production safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
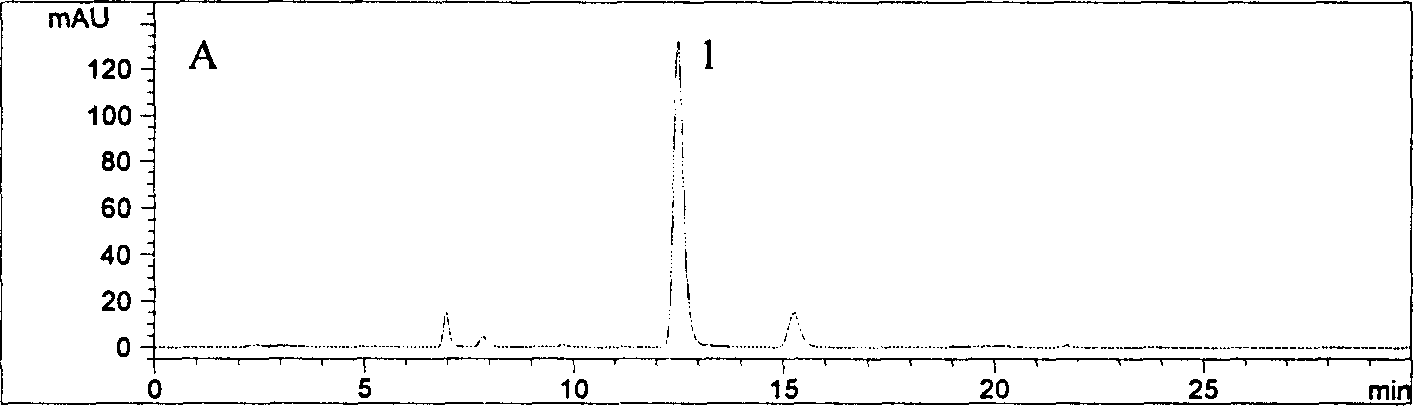
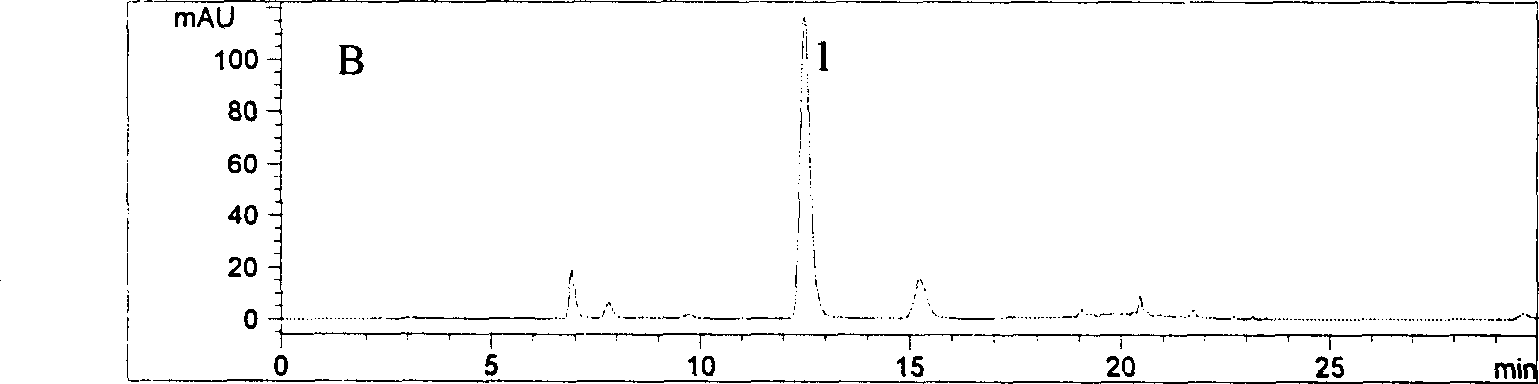
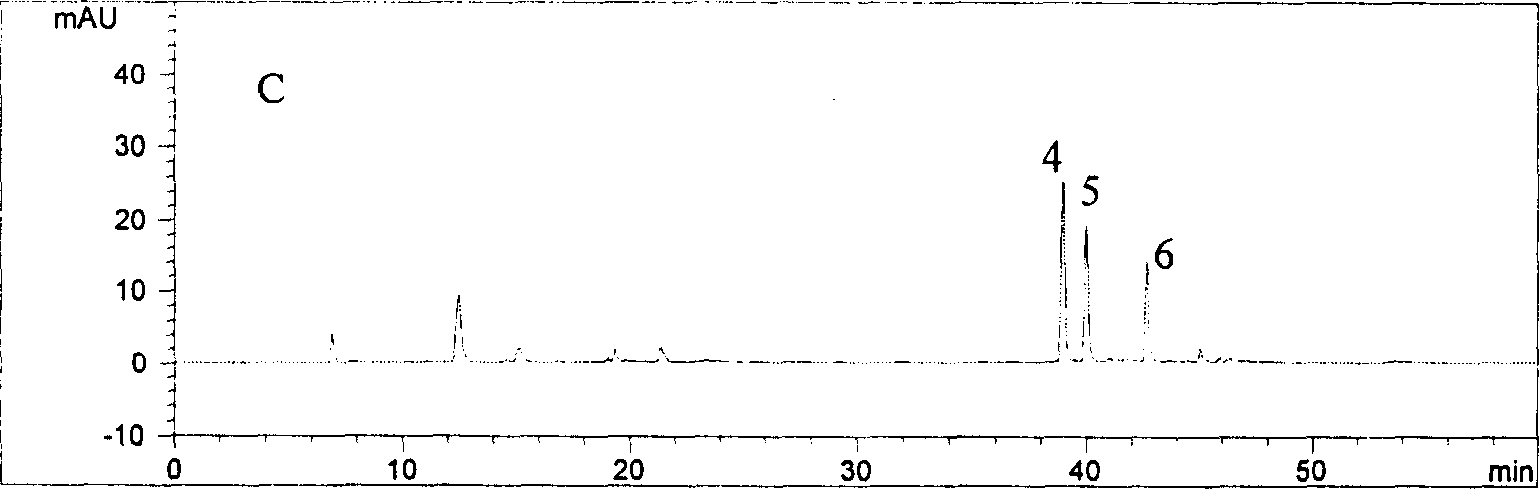
[0031] 1 Assay method
[0032]Instruments and reagents High performance liquid chromatograph Aglient1100 series, binary pump G1312A, autosampler, diode array detector, Chemstation A.06.01 chemistry workstation, chromatographic column: Aglient Zorbax 80A, Extend-C18 (4.6mm×250mm, 5cm ), experimental water (Guangzhou Robust Food and Beverage Co., Ltd.), acetonitrile is chromatographically pure, and phosphoric acid is analytically pure.
[0033] Chromatographic conditions: mobile phase: 0.2% phosphoric acid solution-acetonitrile (88:12), maintained for 0-12min, linearly changed to (83:17) at 12-15min, and maintained the ratio (83:17) at 15-30min; 30-60min linearly changed to (50:50). The flow rate is 1.0mL·min -1 , The detection wavelength is 327nm, and the column temperature is 30°C.
[0034] 2 choice of medicinal materials
[0035] In the honeysuckle genus Lonicera japonicus, L. hypoglauca, L. confusa, L.similes, L. dasystyla, and L. honeysuckle (L. L. chrysantha) can be used to ex...
Embodiment 2
[0054] Take 20kg of Lonicera japonicus leaf medicinal material, pulverize it into 40 mesh powder, extract with petroleum ether at 80℃ for 6 hours to degrease, and extract 8 times the volume of 60% ethanol by the weight of the medicinal material, heat reflux at 85℃ for two times, 1 time each time hour. The extracts were combined, and the solvent was recovered under reduced pressure. The obtained concentrated solution was segmented by D101 macroporous resin, first eluted with water, and then eluted with 10% and 35% ethanol in sequence. Combine water and 10% alcohol eluent, after concentration, first add ethanol to 50% alcohol content, alcohol precipitation, remove the precipitate, the filtrate is concentrated to (70 ℃) relative density of 1.08, then add ethanol to the alcohol content It is 80%, alcohol precipitation, centrifugation to take the supernatant, concentration and vacuum drying to obtain 1.30kg of crude chlorogenic acid with a purity of 70.3%.
[0055] In addition, the 35%...
Embodiment 3
[0058] Take 30 kg of Lonicera japonicus leaf medicinal material, pulverize it into 40 mesh powder, extract with petroleum ether at 85°C for 5 hours by Soxhlet method for degreasing, and extract the medicinal material with 10 times the volume of 75% ethanol under hot reflux for two times, each time for 1 hour. The extract was decompressed to recover the solvent, the concentrated solution was separated by D101 macroporous resin, and directly eluted with 10% ethanol to collect the 10% ethanol eluate; then eluted with 40% ethanol to collect the 40% ethanol eluate. Take 10% ethanol eluate, after concentration, first add ethanol to 50% alcohol content, alcohol precipitation, remove the precipitate, the filtrate is concentrated to (70 ℃) relative density of about 1.10, then add ethanol to the alcohol content of 80 %, alcohol precipitation, centrifugation to take the supernatant, concentration and vacuum drying to obtain 1.9 kg of crude chlorogenic acid with a purity of 72.5%.
[0059] In...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com