Process for derivatizing carbon nanotubes with diazonium species and compositions thereof
A carbon nanotube, single-wall carbon nanotube technology, applied in the direction of carbon nanotubes, single-walled carbon nanotubes, carbon compounds, etc., can solve problems such as obstruction and the existence of a large amount of graphite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
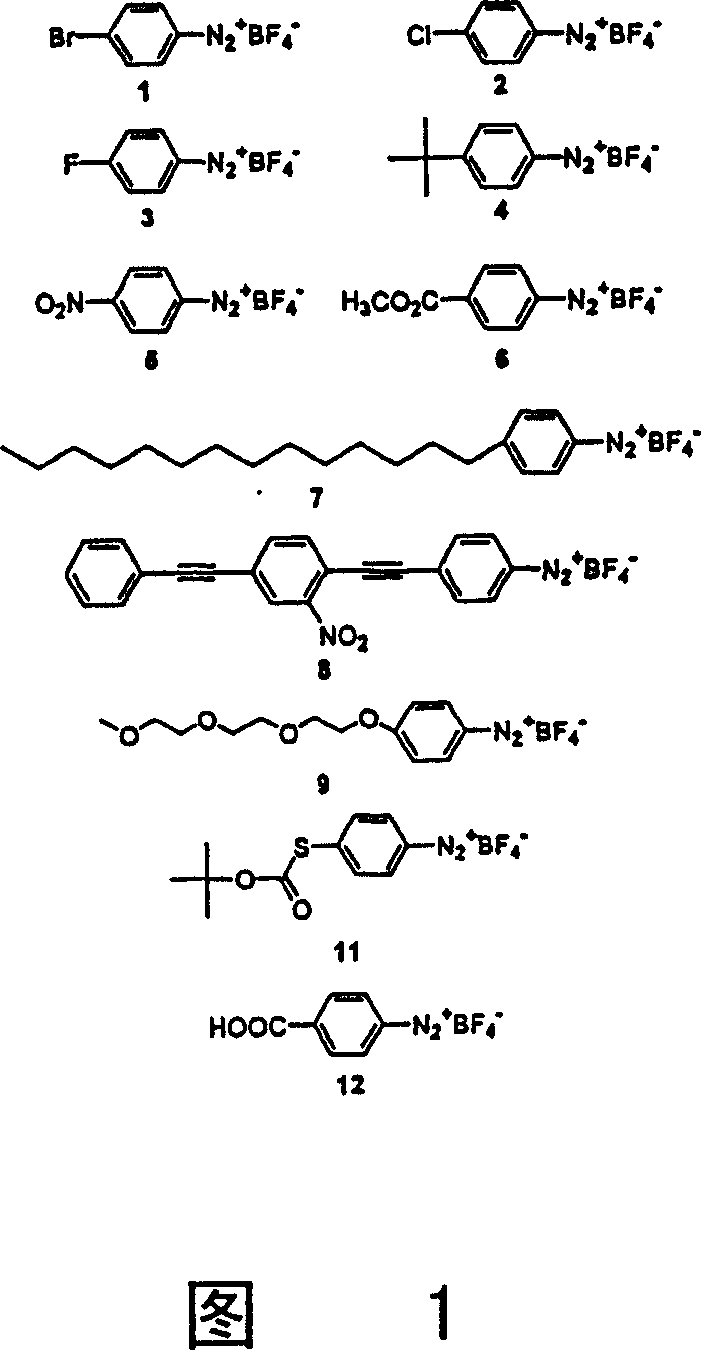
[0050] For the electrochemical derivatization experiments, a piece of "Bucky paper" obtained by filtering the suspension was used as the working electrode of the three-electrode cell, immersed in the acetonitrile solution containing the diazonium salt and the electrolyte. The diazonium salts may be reduced to aryl radicals on the Baker paper surface, which subsequently form covalent bonds with the nanotubes. There is a lot of data on the conductivity of single-walled carbon nanotubes. In general, aryl diazonium salts are readily prepared under conditions that allow for a wide variety of functional groups. Thus, the method described here allows the functionalization of nanotubes with a wide variety of diazonium salts, including those that provide chemical treatments for re-elaboration after attachment to the nanotubes.
[0051] The purified single-walled nanotubes (herein referred to as SWNT-p) employed in this study contained little amorphous carbon or other exotic carbon imp...
Embodiment 12-17
[0106] The nanotubes used in this study are still prepared by the gas-phase catalytic method developed by Smalley et al. and are now commercially available (Carbon Nanotechnology, HiPco Materials). The method for the purification of production raw materials is to oxidize in humid air at 250°C for 24 hours, then stir in concentrated hydrochloric acid at room temperature for 24 hours, the formed material is first washed with a large amount of water, then with 10% aqueous sodium bicarbonate solution, Wash again with water. After vacuum drying, the material was ready for functionalization reactions.
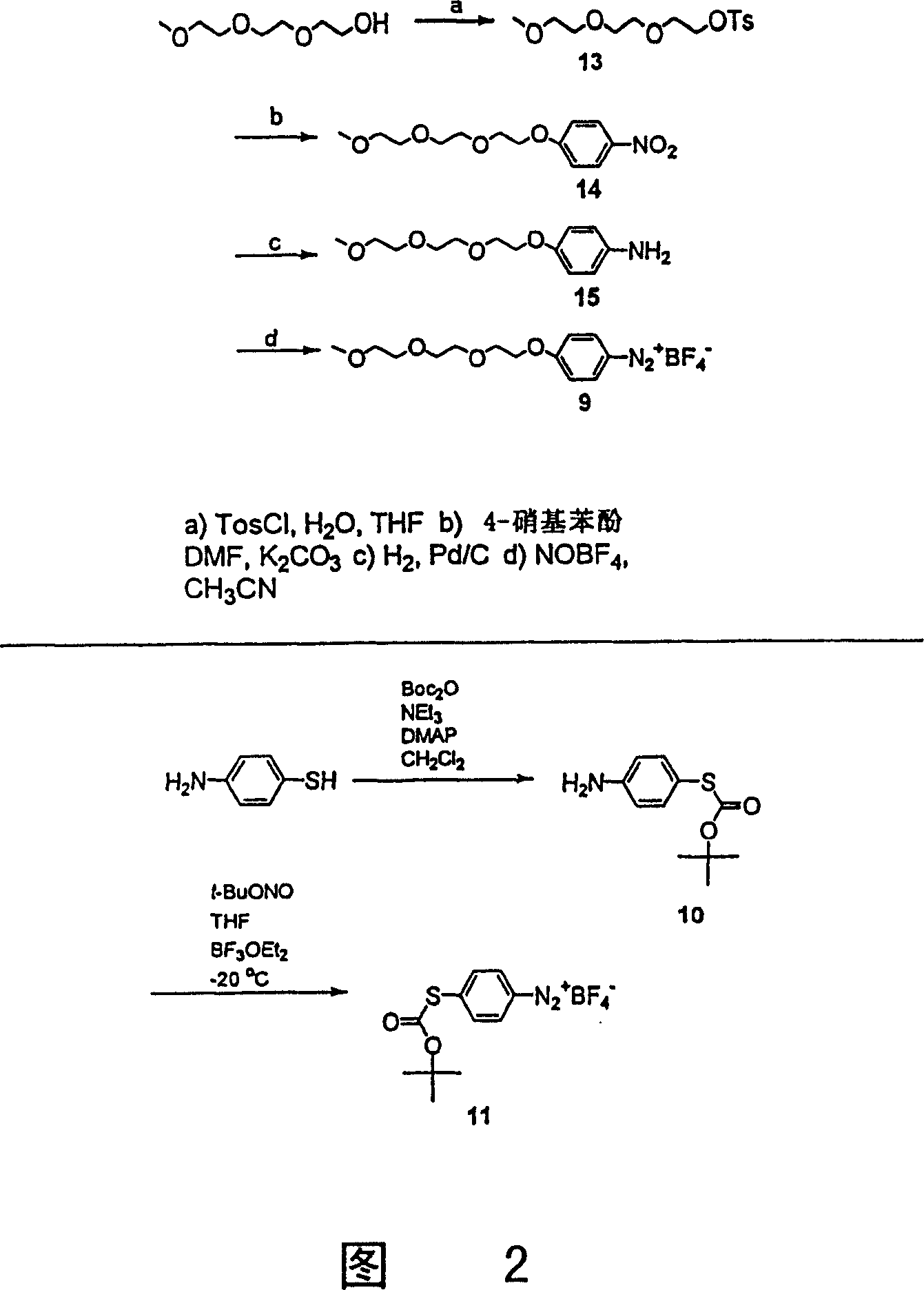
[0107] Figure 12 shows the sequence of reactions. In a typical test, about 8 mg of single-walled carbon nanotubes were placed in 10 ml of 1,2-dichlorobenzene (ODCB) and ultrasonically oscillated for 10 minutes. Add the solution of aniline derivative (2.6mmol, about 4 g equivalent / mole carbon) in 5ml of acetonitrile to this suspension, transfer the feed solution to the reaction tube...
Embodiment 18
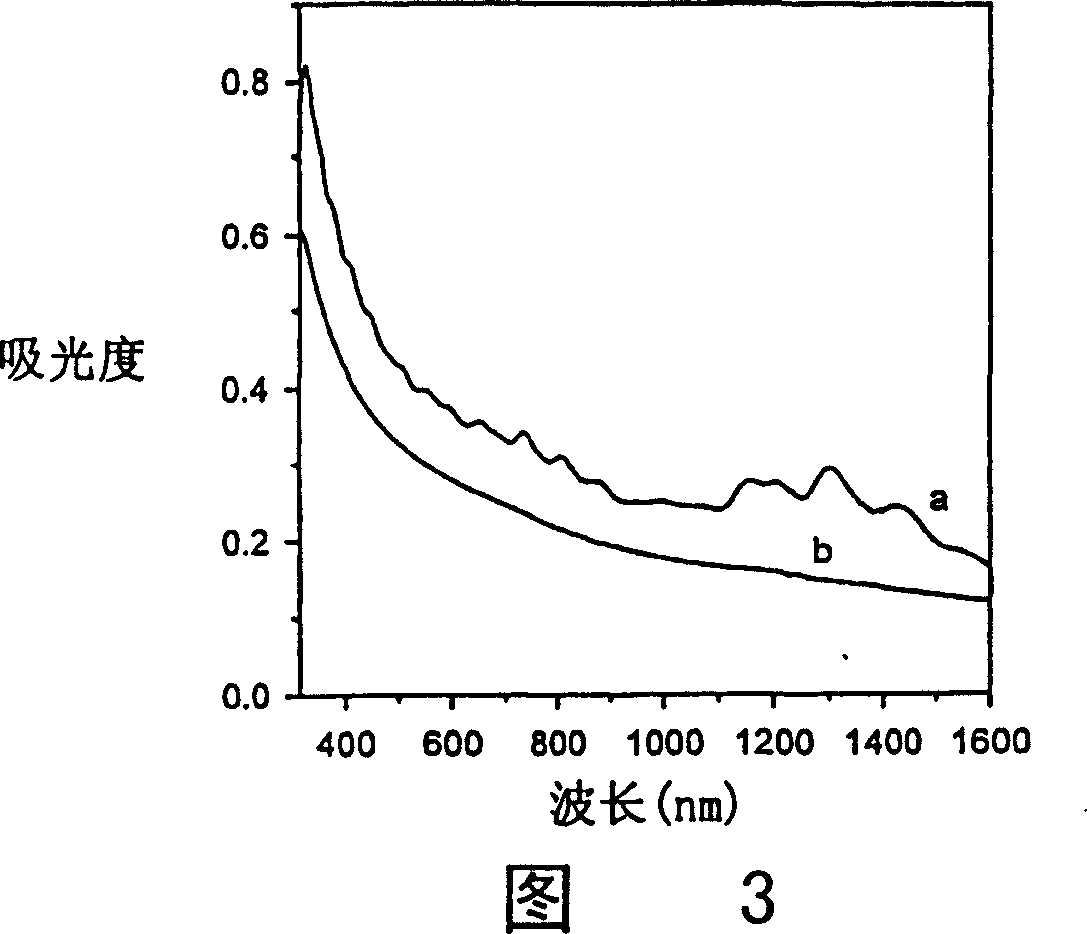
[0119] Derivatization reactions with aryldiazonium salts can also be induced photochemically. The photochemical reaction can also be carried out with the tetrafluoroborate 4-chlorobenzene diazonium salt adopted and prepared in Example 2. Therefore, a suspension of SWNT-p in 1,2-dichlorobenzene was generated by sonication. To this suspension was added a portion of the diazonium salt dissolved in a minimal amount of acetonitrile. The obtained mixed solution is placed in a photochemical reaction chamber for stirring. The excitation wavelength is 254nm (ultraviolet light source). The light source used for the photochemically induced reaction can be light of any wavelength, usually ultraviolet light or visible light. Figure 15 shows this reaction. The resulting material was similar in all respects to SWNT-2 prepared electrochemically according to the present invention.
[0120] This experiment further confirms that the reaction of diazonium salt leads to covalent attachment to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com