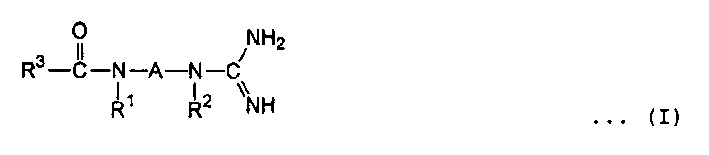Fungicide, composition thereof, use and fungicidal method thereof
A technology of bactericide and composition, which is applied in the field of inhibiting microbial reproduction, and can solve the problems of not using preservatives or bactericides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] The present invention is further illustrated by way of comparison and examples.
[0115] (1) Synthesis of amidoguanidine-containing derivatives
[0116] 180 g of water and 110 g of 2-propanol were added to 52.7 g (0.23 mol) of 4-aminobutylguanidine sulfate. The pH of the system was adjusted to 11.0 with 27% sodium hydroxide aqueous solution, and the temperature was adjusted to 10°C. In this system, 50.3 g (0.23 mol) of lauroyl chloride was added dropwise within 35 minutes. During this period of time, the temperature of the system was maintained at 8-12°C, and the pH value was maintained at 10.9-11.0 with a 27% aqueous sodium hydroxide solution. After completion of the dropwise addition, the reaction further matured during 30 minutes.
[0117] After the reaction was completed, the temperature of the system was adjusted to 50°C. The obtained oil phase was separated, the pH was adjusted to 14 with a 27% sodium hydroxide aqueous solution, and cooled to room temperature. After c...
Embodiment 2~5
[0137] In order to evaluate the contribution of the acyl moiety to the antibacterial activity in the amidoguanidine-containing derivatives, 4-guanidinobutyl decacoamide acetate (DAG-AcOH) and 4-guanidinobutyl dodecylamide acetic acid were tested Salt (LAG-AcOH), 4-guanidinobutyl tetradecyl amide acetate (MAG-AcOH), 4-guanidino butyl hexadecyl amide acetate (PAG-AcOH) in each experimental strain Their growth is completely inhibited by their concentration (MIC).
[0138] The experimental strains used are Escherichia coli (ATCC8739), Pseudomonas aeruginosa (ATCC9027), Staphylococcus aureus (ATCC6538), Streptococcus mutans (ATCC35275), Propionibacterium acnes (ATCC6919), Candida albicans (ATCC10231) , Aspergillus niger (ATCC16404), Trichophyton mentagrophytes (ATCC11480), Malassezia saccharomyces (ATCC14521), MRSA and Cladosporium cladosporioides.
[0139] The bacterial cell suspensions of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans mutants, P...
Embodiment 6 and 7
[0153] Example 6
[0154] Example 8
[0155] Prepare the antibacterial cream with the formula shown in Table 7 below (based on the weight% of the net components, and the total weight is 100%). When each of the antibacterial creams thus prepared was used, satisfactory performance was obtained.
[0156] Table 7:
[0157] Example 11
[0158] Example 13
[0159] Example 16
[0160] Example 21
[0161] Example 23
[0162] Example 26
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com