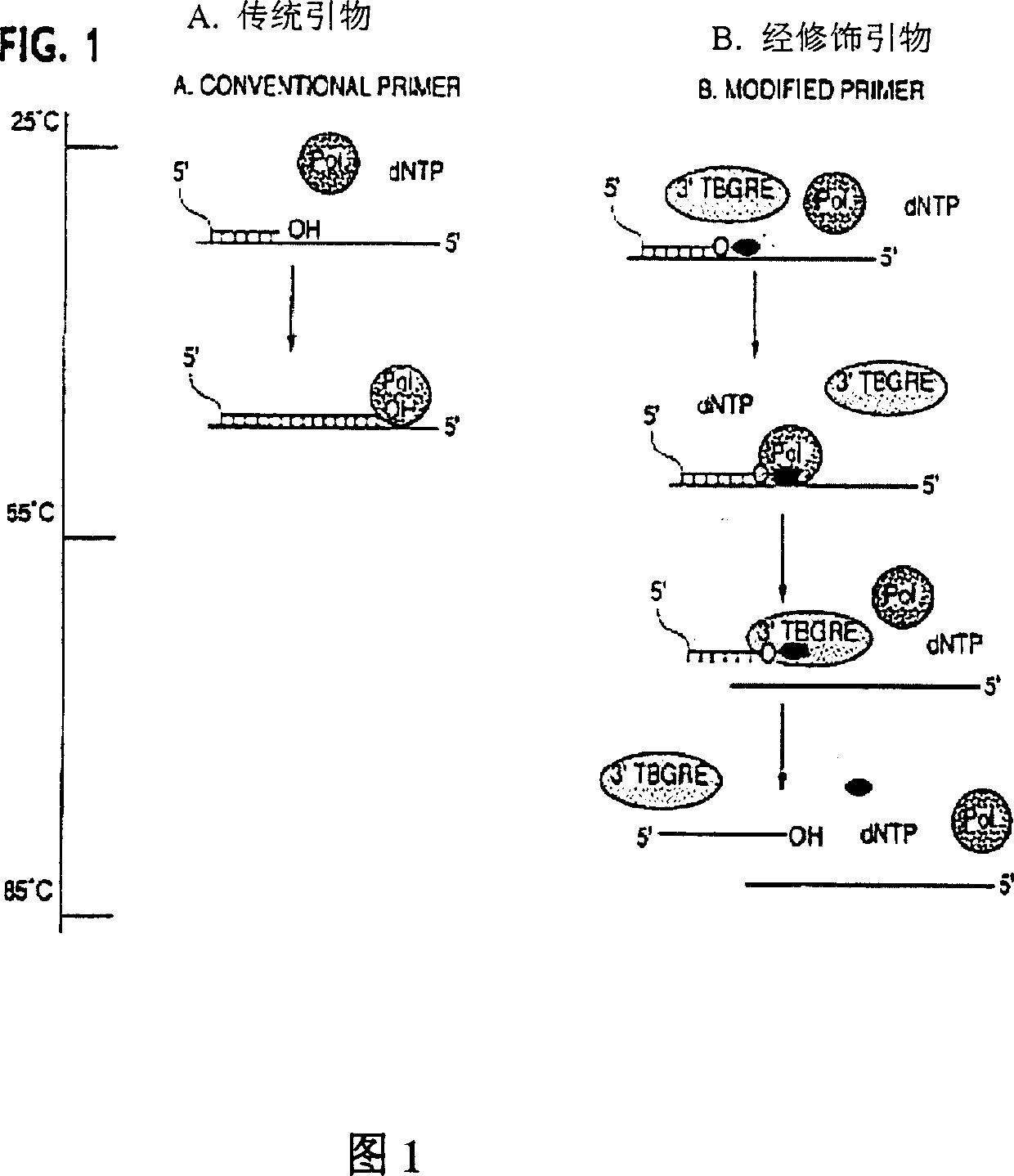
Method and compositions for improved polynucleotide synthesis
A technology of polynucleotides and nucleosides, which is applied in the direction of microorganism-based methods, plant genetic improvement, chemical instruments and methods, etc., and can solve problems such as temperature sensitivity of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0099] 3′polynucleotide phosphatase activity detection
[0100] 3'phosphate-modified oligonucleotides were chemically synthesized (Midland Certified Reaget Company, Midland, Tex.). The oligonucleotide sequence is composed as follows: 5'-GCT GCTCTGTGCATCCGAGTGG-p-3' (SEQ ID No: 7)
[0101] T4 polynucleotide kinase without 3' polynucleotide phosphatase activity 32 The P-label is at the 5' end of the oligonucleotide (Yang, S.W., et al. Proc. Natl. Acad. Sci. U.S.A. 93(21):11534-9 (1996)). with 32 P-labeled oligonucleotides were mixed with purified 3′ polynucleotide phosphatase in 1×PCR reaction buffer (10Mm Tris-HCl, pH8.3, 50mM MgCl 2) at 72°C for 5 minutes. Add DNA sequencing buffer, heat at 90° C. for 2 minutes, and analyze the sample by 12% polyacrylamide-7M urea gel electrophoresis. Figure 4 is an autoradiogram of samples quantified with PhophorImager (Molecular Dynamics). One unit of 3' phosphatase is defined as the ability to remove 5 μmol of the 3' terminal phosphat...
example 2
[0102] Isolation and Purification of 3' Phosphatase from Pfu
[0103] 100 g wet weight of Pfu cells (purchased from the Marine Biotechnology Center at the University of Maryland, Baltimore, Md) were suspended in lysate, 20 mM Tris-HCl, Ph7.5, 1 mM EDTA, 1 mM DTT, 0.2 M NaCl on ice , 10 mM mercaptoethanol and 2 mM benzylsulfur fluoride. Cells were then lysed by ultrasound and centrifuged at 200 rpm for 10 minutes on a Sorvall GS-3 rotor 8. Add 0.05 vol of 10% polyethyleneimine solution to the supernatant, mix well and centrifuge, then fractionate with ammonium sulfate (45-80% saturation). Then use phosphocellulose column (P-11; Whatman, Inc.; activity eluted.apprxeq.0.6M NaCl), Source 15S (Pharmacia; activity eluted.apprxeq.0.2M NaCl), double-stranded DNA-cellulose (Sigma; activityeluted.apprxeq.0.15M NaCl), Mono S column (Pharmacia; activityeluted.apprxeq.0.35M NaCl) and heparin agarose, Mono Q column (Pharmacia; activityeluted.apprxeq.0.25M NaCl) chromatography, finally in ...
example 3
[0105] Peptide sequencing of the N-terminus of 3 phosphatases
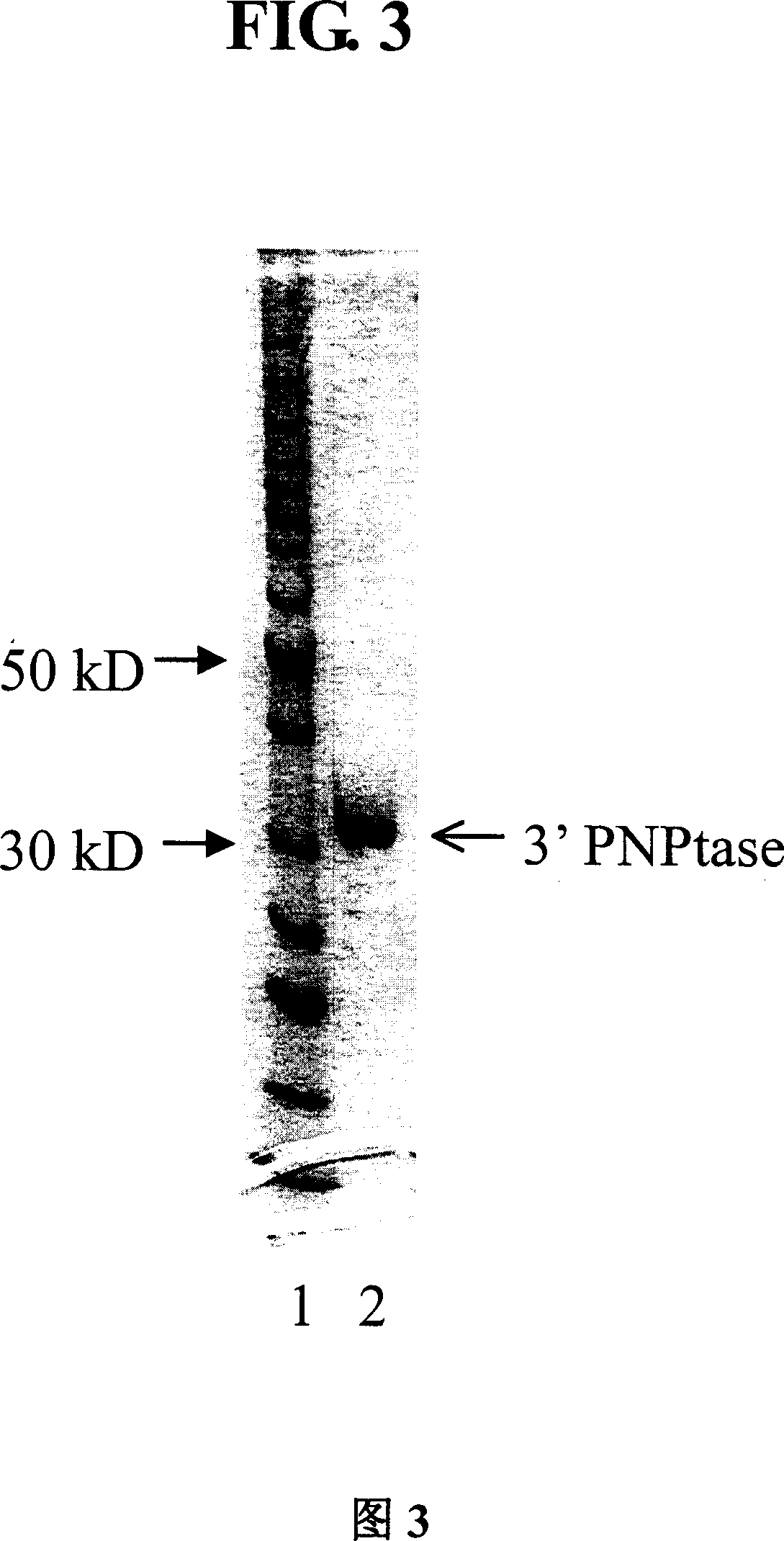
[0106] Part of the purified 3' polynucleotide phosphatase was added to 7% SDS-Tricine PAGE. The protein in the SDS gel was electrotransferred to PVDF membrane (Immobolin-P from Millipore) in transfer buffer (0.5.times.TBE (pH8.4), 20% methanol, 0.5mMEDTA), 0.5A electrophoresis, transfer 1 hour. Membranes were stained with Coomassie Brilliant Blue R-250 for 10 minutes and destained twice with 100% methanol. The band corresponding to the 3' polynucleotide phosphatase on the membrane was excised. Its amino acid sequencing was done with Yale University commercial facilities. A sequence of 27 amino acids is listed in single letters starting from the N-terminus: FKIDRLRFGTAGIPLSTPKPSTIAGI (SEQ ID NO: 1). Example 4
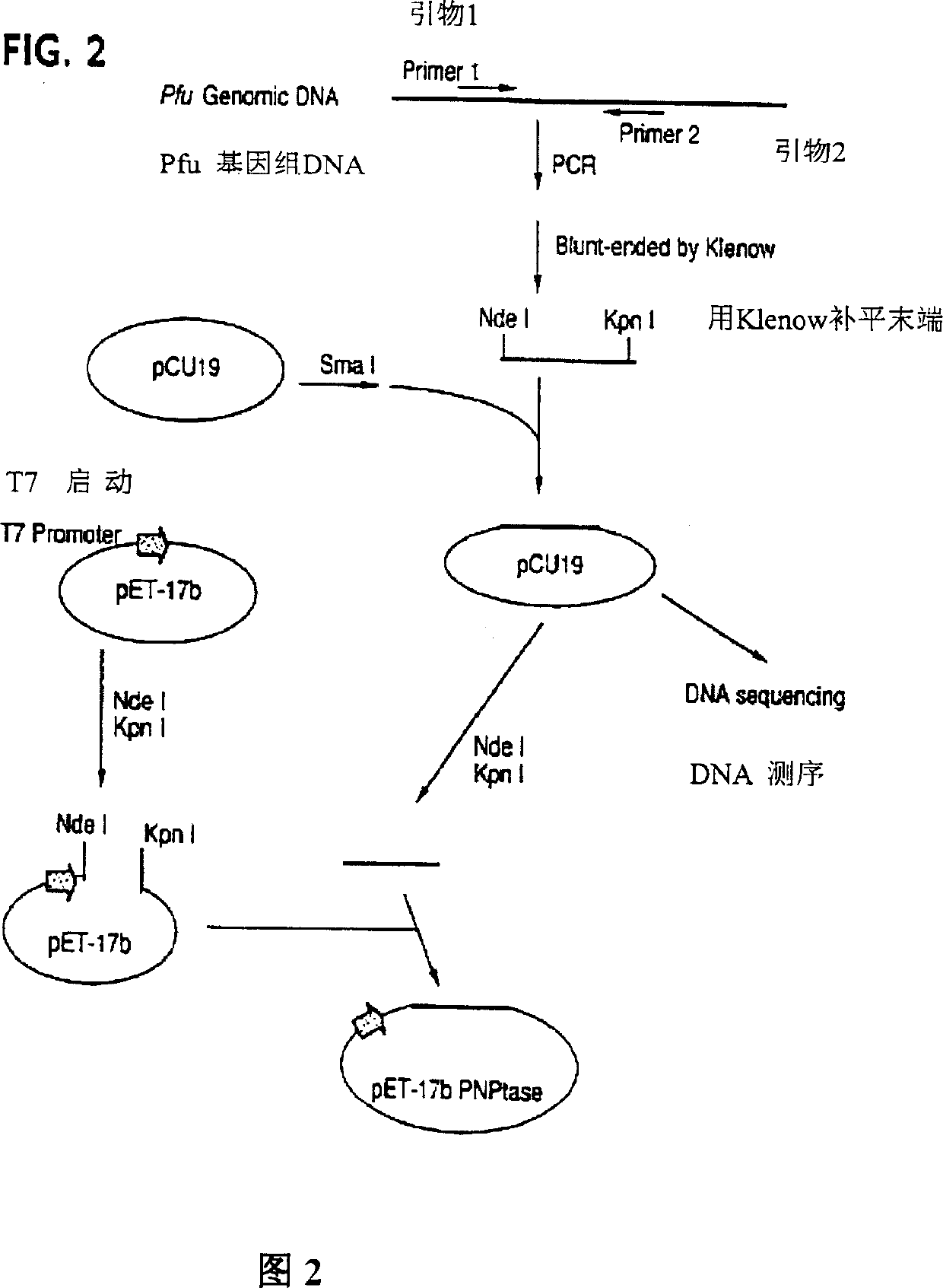
[0107] Cloning of the gene encoding the 3' phosphatase from the Pfu genome
[0108] Figure 2 is the cloning process of the 3' phosphatase gene, which is briefly described as follows: use the BLAST progr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com