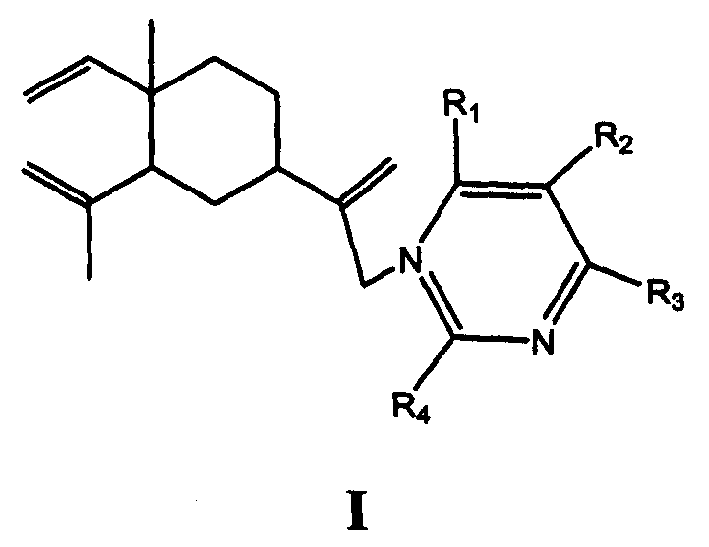
Miazines ramification of š�-elemene and its synthetic method
A technology for elemene and derivatives, applied in the field of organic compound β-elemene, can solve problems such as poor water solubility and high toxicity, and achieve the effects of mild reaction conditions, simple synthesis method and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Step 1, β-elemene reacts with hypochlorite in acetic acid to prepare the halogenated product intermediate
[0026] In a 100mL round bottom bottle equipped with an electromagnetic stirrer, add 10.2g of β-elemene and 10ml of glacial acetic acid, and control the reaction temperature at about 0-5°C. Add 40ml of sodium hypochlorite (containing 8.6% available chlorine) dropwise from a constant pressure dropping funnel under vigorous stirring, and pay attention to maintaining the temperature of the reaction system not exceeding 5°C, and complete the addition within 4 hours. Stirring was continued at this temperature for 1 hour, then the reaction mixture was poured into a separatory funnel, extracted 3 times with petroleum ether (3×60 ml), and the organic phases were combined and dried over anhydrous sodium sulfate. After the organic phase was concentrated, a yellow oil was obtained, and the β-elemene chloride was obtained by silica gel column chromatography, which was used for...
Embodiment 2
[0030] Step 1. FeCl 3 Treatment of other transition metals
[0031] Dissolve 5g of ferric chloride in 10mL of acetone, then add 20g of silica gel, remove acetone under reduced pressure on a rotary evaporator, then place the silica gel in an oven, dry at 120°C for 3-4 hours, and cool Store in a desiccator for later use. NiCl was prepared in the same way 2 , CoCl 2 catalyst. IrCl 3 The preparation method is to IrCl 3 Dissolve in 20ml of water, then distill off most of the water under reduced pressure, place the obtained powder in an oven, dry at 140°C for 4-5 hours, cool naturally and place in a desiccator for later use.
[0032] Step 2, β-elemene uses hypochlorite to prepare halogenated intermediates under the catalyzed reaction of transition metal chlorides
[0033] In a 100ml round bottom bottle equipped with an electromagnetic stirrer, add 10.2g β-elemene, 50ml n-hexane, FeCl 3 50% moL), cooled to below 0°C, and 40ml of sodium hypochlorite (containing 8.6% of availab...
Embodiment 3
[0037] Step 1. FeCl 3 The processing such as transition metal is the same as embodiment 2 step 1.
[0038] Step 2, β-elemene uses NBS to prepare β-elemene bromide intermediate under transition metal chloride catalyzed reaction
[0039] In a 100mL round bottom bottle equipped with an electromagnetic stirrer, add 10.2g of β-elemene, 30mL of ether, and 10% moL of cobalt dichloride, cool to below 0°C, add 10g of NBS in batches under vigorous stirring, and react 4 After 1 hour, the reaction solution was filtered, washed with n-hexane, and then the organic phase was concentrated and subjected to fast silica gel column chromatography, using petroleum ether as the eluent to remove inorganic salts and succinimide generated by the reaction to obtain unreacted β -Elemene and β-elemene bromide, the mixture was used directly in the reaction of step 3. It is also possible to obtain a relatively pure β-elemene bromide for the reaction of step 3 by further column chromatography.
[0040] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com