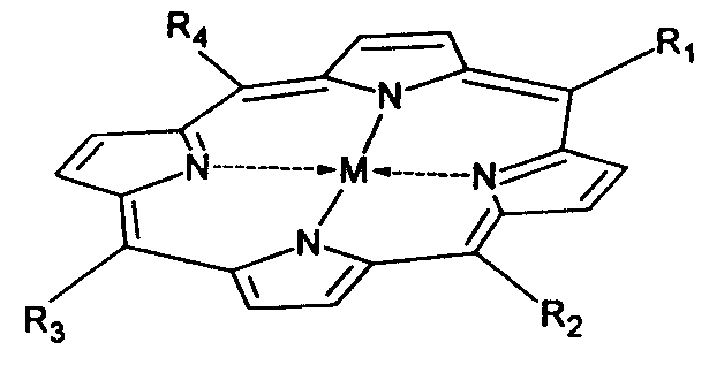
Substituted carbolnie metal complex and application thereof
A coordination compound and metal coordination technology, which is applied to compounds of elements of group 2/12 of the periodic table, compounds containing elements of group 8/9/10/18 of the periodic table, organic chemistry, etc., can solve the problem of cathode performance Attenuation, catalytic active components and unknown structure, unclear mechanism, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of meso-5,10,15,20-tetra(p-methoxyphenyl)porphyrin
[0061] Add 30-60ml of propionic acid, 20-30ml of butyric acid, 20-30ml of nitrobenzene, and 20-30ml of glacial acetic acid into a three-necked flask equipped with a stirrer, a water separator, and a reflux condenser, and heat to reflux for 30-60min at 5 Add dropwise 40mmol p-methoxybenzaldehyde in 30~60ml propionic acid solution within ~30min, add dropwise 40mml (5.36g) freshly steamed pyrrole in 30~60ml nitrobenzene solution within 10~30min, the solution turns from yellow to Purple, brown, brown to black. Heated to reflux for 0.5-2h to obtain a black solution, left to cool overnight, and filtered with suction to obtain a black powder. Wash with secondary water and absolute ethanol to obtain blue-black crystals.
[0062] The crude product was subjected to column chromatography, the column was filled with silica gel, and dichloromethane was used as eluent, and the pure product was obtained...
Embodiment 2
[0063] Embodiment 2: the synthesis of other mesosymmetric four-substituted phenyl porphyrins and
[0064] The purification of the crude product adopts different methods according to the different properties of the substituents. In general, there are several methods: (1) column chromatography; (2) choose a suitable solvent for recrystallization; (3) choose a suitable solvent pyrolysis After freezing and crystallization, etc.
Embodiment 3
[0065] Embodiment 3: the synthesis of meso-5,10,15,20-tetra(p-methoxyphenyl)porphyrin iron (II) complexes
[0066] Solid-phase synthesis method: Mix 0.1 mol of meso-5, 10, 15, 20-tetrakis(p-methoxyphenyl) porphyrin with 0.12 mol of ferrous ammonium sulfate, grind in a special grinder for 20-50 minutes, and use Wash with hot dilute acetic acid three times to remove excess metal salts. The crude product is separated by column chromatography, using neutral alumina or silica gel to fill the column, and using CH 2 Cl 2 , a mixed solvent of petroleum ether for elution to obtain the desired components. The pure product was obtained after three column passes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com