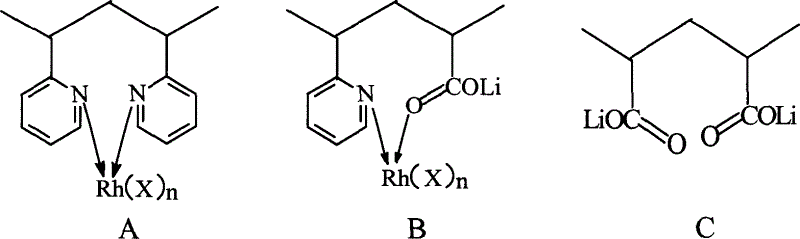
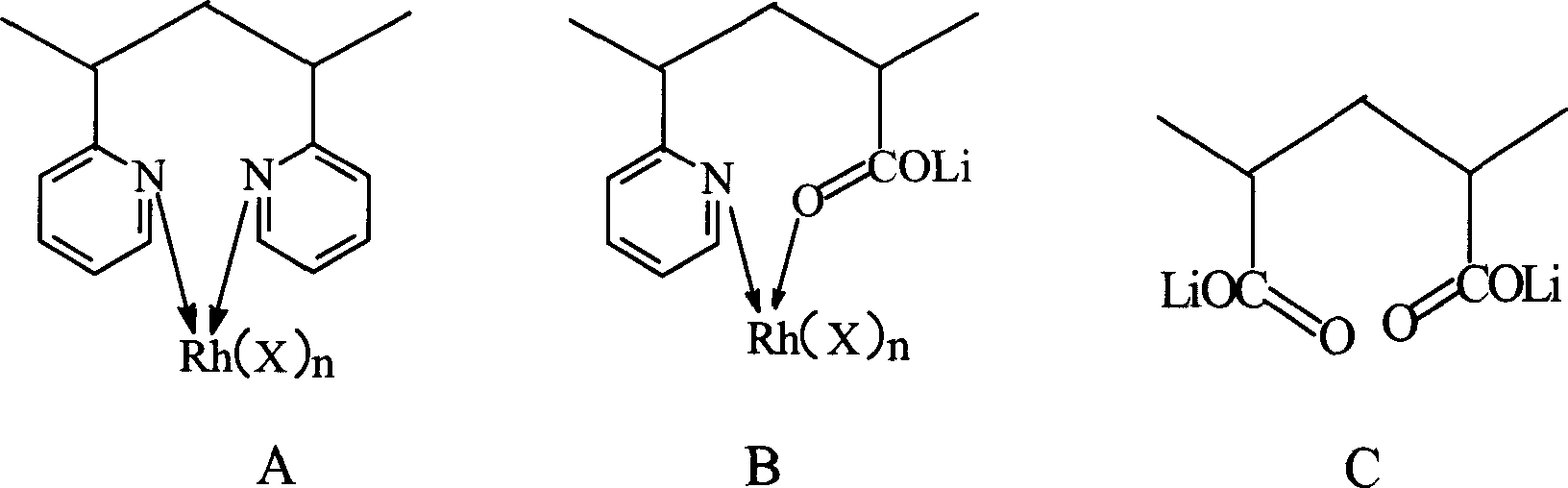
Copolymer ligand rhodium-lithium bimetal catalyst and its manufacturing method and application
A bimetallic catalyst and copolymer technology, applied in organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, carbon monoxide reaction to prepare carboxylic acid, etc., can solve the problem of increasing the corrosion of reaction equipment and no industrial application Value, material requirements and other issues, to achieve the effect of avoiding water gas reaction, excellent stability, high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 0.3mol of purified 2-vinylpyridine and 0.7mol of methyl acrylate to 6mmol of azobisisobutyronitrile, mix well and add to 2mol of benzene, heat to 60°C for 24 hours, precipitate with ether, filter, Wash and dry to obtain the copolymer.
[0035] Add the copolymer into 400ml aqueous solution containing 10wt% sodium hydroxide, reflux and stir for 1h, adjust the pH to 6 with hydrochloric acid, stir and precipitate with ether after cooling, continue to wash the precipitate with water, salt out, filter and dry, then add Put it into an aqueous solution containing 0.47 mol of lithium hydroxide, heat it at 60°C and stir for 2 hours, precipitate it with ether after cooling, dissolve it in methanol, precipitate it with ether, and dry it to obtain a copolymer lithium salt ligand.
[0036]Dissolve the copolymer lithium salt ligand in 1.5mol methanol solution, stir at 30°C for 10min, add dropwise methanol solution containing 13mmol rhodium acetate, react for 30min, and after the s...
Embodiment 2
[0038] Weigh 0.5mol of purified 4-vinylpyridine and 0.5mol of maleic anhydride monomer, add 4mmol of benzoyl peroxide as an initiator, react in 2.5mol of benzene at 60°C for 20h, precipitate with ether, and filter , washing and drying to obtain the copolymer.
[0039] The copolymer was refluxed and hydrolyzed with 500ml aqueous solution containing 5wt% potassium hydroxide for 2 hours, adjusted the solution to pH 5 with hydrochloric acid, stirred and precipitated with ether after cooling, continued to wash the precipitate with water, salted out, filtered and dried, and then added to a solution containing 0.3 In an aqueous solution of mol lithium hydroxide, react at 70°C for 2 hours, precipitate with ether, dissolve in methanol, precipitate with ether and dry to obtain the ligand of the copolymer lithium salt.
[0040] Dissolve the copolymer lithium salt ligand in a mixture of 1.5mol methanol and 0.5mol acetic acid, stir at 20°C for 20min, add an aqueous solution containing 39mm...
Embodiment 3
[0042] Mix 0.3 mol of purified 2-vinylpyridine, 0.5 mol of methyl acrylate and 0.2 mol of methacrylate diester monomer, add 10 mmol of azobisisobutyronitrile initiator, then add 60 ml of kerosene, and mix well , added to the filled with 10wt% Na 2 SO 4 In the reaction flask of the aqueous solution, Na 2 SO 4 The volume of the aqueous solution is 400ml, the temperature is raised to 70°C, the rotation speed is 400 rpm, the amount of bentonite is 50g, and the amount of bentonite is 50g. After stirring for 1 hour, reduce to 200 rpm, stop after 24 hours of continuous reaction, and filter After washing with water, extract with acetone and dry to obtain high molecular polymer.
[0043] Add the polymer to an aqueous solution of sodium hydroxide and react at 100°C for 2 hours, adjust to an acidic pH of 5.5 with hydrochloric acid, stir and precipitate with ether after cooling, continue to wash the precipitate with water, salt out, filter and dry, and then add to a solution containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com