Production method of potassium dichromate
A technology of potassium dichromate and production method, applied in directions such as chromate/dichromate, can solve the problems of low utilization rate of chromium in chromite, environmental pollution, unreusable by-products, etc., and achieve the utilization rate of chromium High, the effect of avoiding chromium mist pollution and eliminating Cl pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
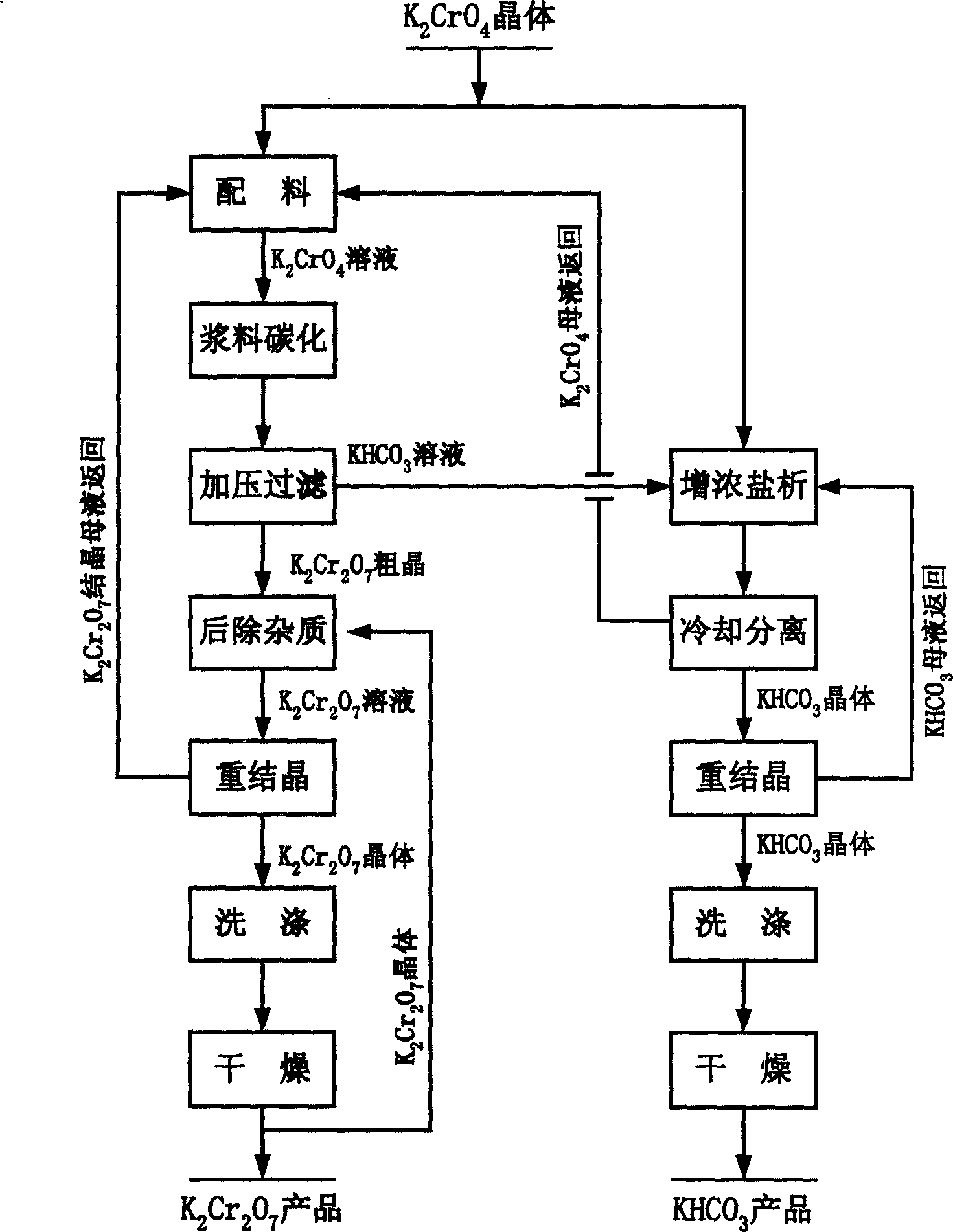
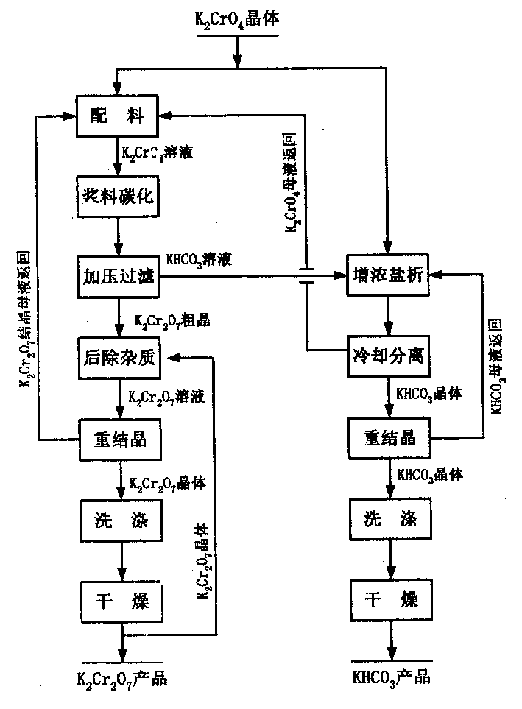
[0041] The reaction process is as figure 1 As shown, the potassium chromate used is an analytical reagent produced by Beijing Hongxing Chemical Factory, and potassium dichromate is an analytical reagent produced by Beijing Hongxing Chemical Factory, CO 2 It is cylinder gas (content ≥99%), and a 1.2-liter autoclave is used for carbonization reaction.
[0042] 1) Prepare 800ml of 550g / L potassium chromate slurry solution. Continuous access to CO 2 The gas is heated to 0.3MPa, and the slurry carbonation reaction is carried out with stirring at a speed of 900 rpm / min. The initial temperature of the reaction is 50°C. The temperature is lowered slowly by indirect cooling with conventional cooling water. After the reaction is controlled for 2 hours, the temperature is lowered to 15°C after the reaction is completed. , The carbonization rate reaches 81.7%. Filter the mixed solution at a pressure of 0.2 MPa to obtain potassium dichromate crystals and potassium bicarbonate solutions, respec...
Embodiment 2
[0046] The reaction process is as figure 1 As shown, the potassium chromate used is an industrial grade potassium chromate product of Henan Zhenxing Chemical Group, and potassium dichromate is an analytical reagent produced by Beijing Hongxing Chemical Plant. CO 2 It is cylinder gas (content ≥99%), and a 1.2-liter autoclave is used for carbonization reaction.
[0047] 1) Prepare 800ml of 700g / L potassium chromate slurry solution. Continuous access to CO 2 Gas to 0.8MPa, stirring at a speed of 1100 revolutions per minute for slurry carbonation reaction. The initial temperature of the reaction is 60°C. Use conventional cooling water to cool down slowly and react for 4 hours. After the reaction is completed, the temperature is reduced to 20°C. , The carbonization rate reaches 84.4%. The mixed solution was filtered at a pressure of 0.4MPa to obtain potassium dichromate crystals and potassium bicarbonate solutions, respectively.
[0048] Item
[0049] 3) The potassium bicarbo...
Embodiment 3
[0051] The reaction process is as figure 1 As shown, the potassium chromate used is an industrial grade potassium chromate product of Henan Zhenxing Chemical Group, and chromic anhydride is an industrial grade product of Henan Zhenxing Chemical Group, CO 2 It is cylinder gas (content ≥99%), and a 1.2-liter autoclave is used for carbonization reaction.
[0052] 1) Prepare 800ml of 650g / L potassium chromate slurry solution. Continuous access to CO 2 The gas is heated to 0.5MPa, and the slurry carbonation reaction is carried out with stirring at a speed of 1000 revolutions per minute. The initial temperature of the reaction is 55°C. Use conventional cooling water to cool down slowly and react for 3 hours. After the reaction is completed, the temperature is reduced to 18°C. , The carbonization rate is 83.1%. The mixed solution was filtered at a pressure of 0.3MPa to obtain potassium dichromate crystals and potassium bicarbonate solutions, respectively.
[0053] Item
[0054]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com