Recombinant 1 type herpes simplex virus and its preparation method and uses
A herpes simplex virus and virus technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve problems such as inability to detect intuitively, complex preparation methods, etc., and achieve strong targeting and good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
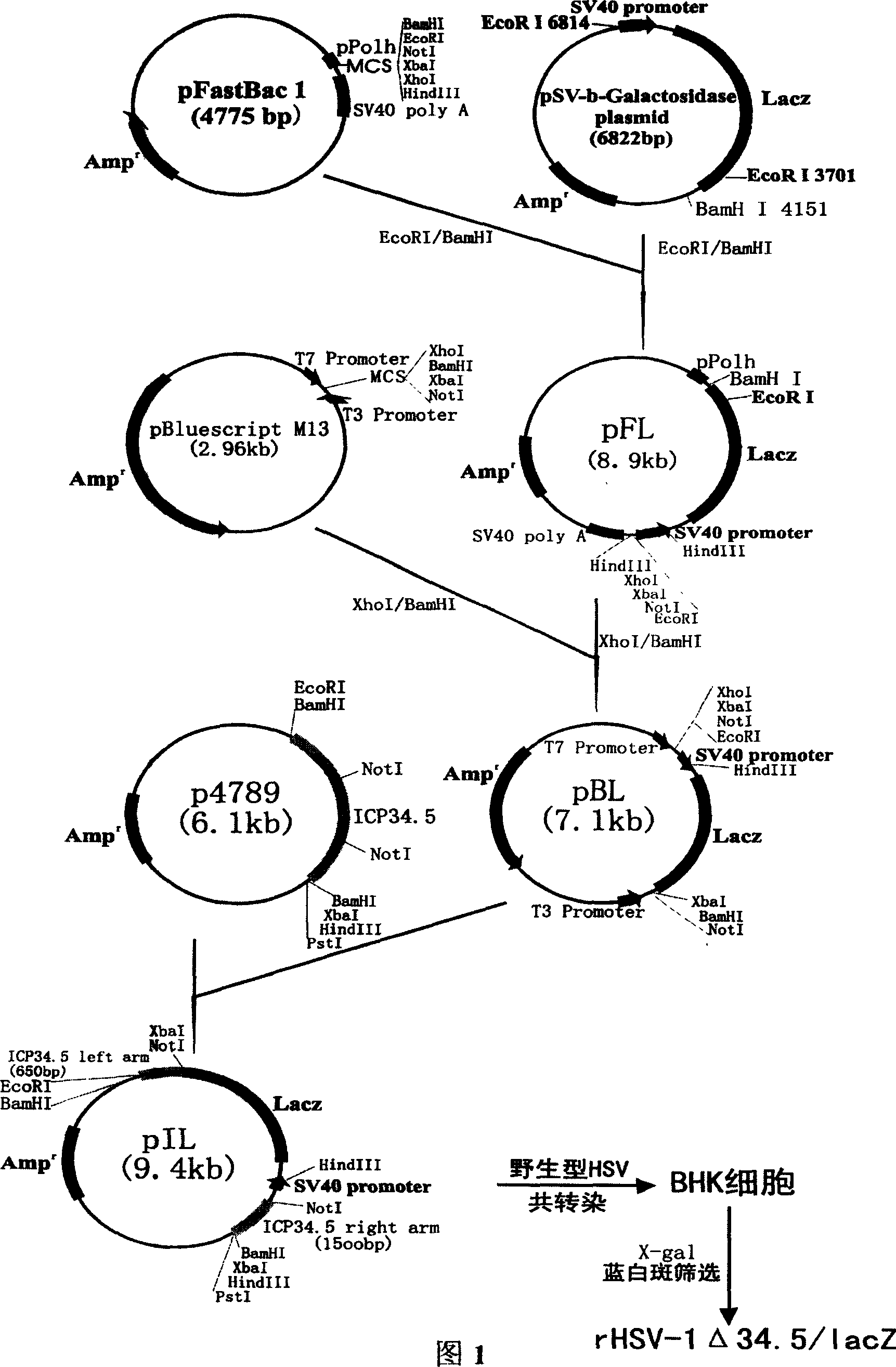
[0054] Example 1 Construction and acquisition of recombinant virus rHSV-1Δ34.5 / lacZ
[0055] The plasmid vector pSV-β-Galactosidase was purchased from Promega Company, and the transposable vector pFastBac1 was purchased from Gibco Company. The vector pSV-β-Galactosidase contains a lacZ expression cassette, and the SV40 promoter controls its expression. Plasmid vector pBluescript-M13 was purchased from Stratagene Company. Plasmid p4789 was donated by Professor Roizman B of the University of Chicago and contains the complete HSV icp34.5 gene and its flanking sequences. The Syrian hamster kidney cell line BHK21 was donated by researcher Gong Zhenkui of Hubei Academy of Medical Sciences, and the wild virus HSV-1 F strain was donated by Professor B. Roizman of the University of Chicago.
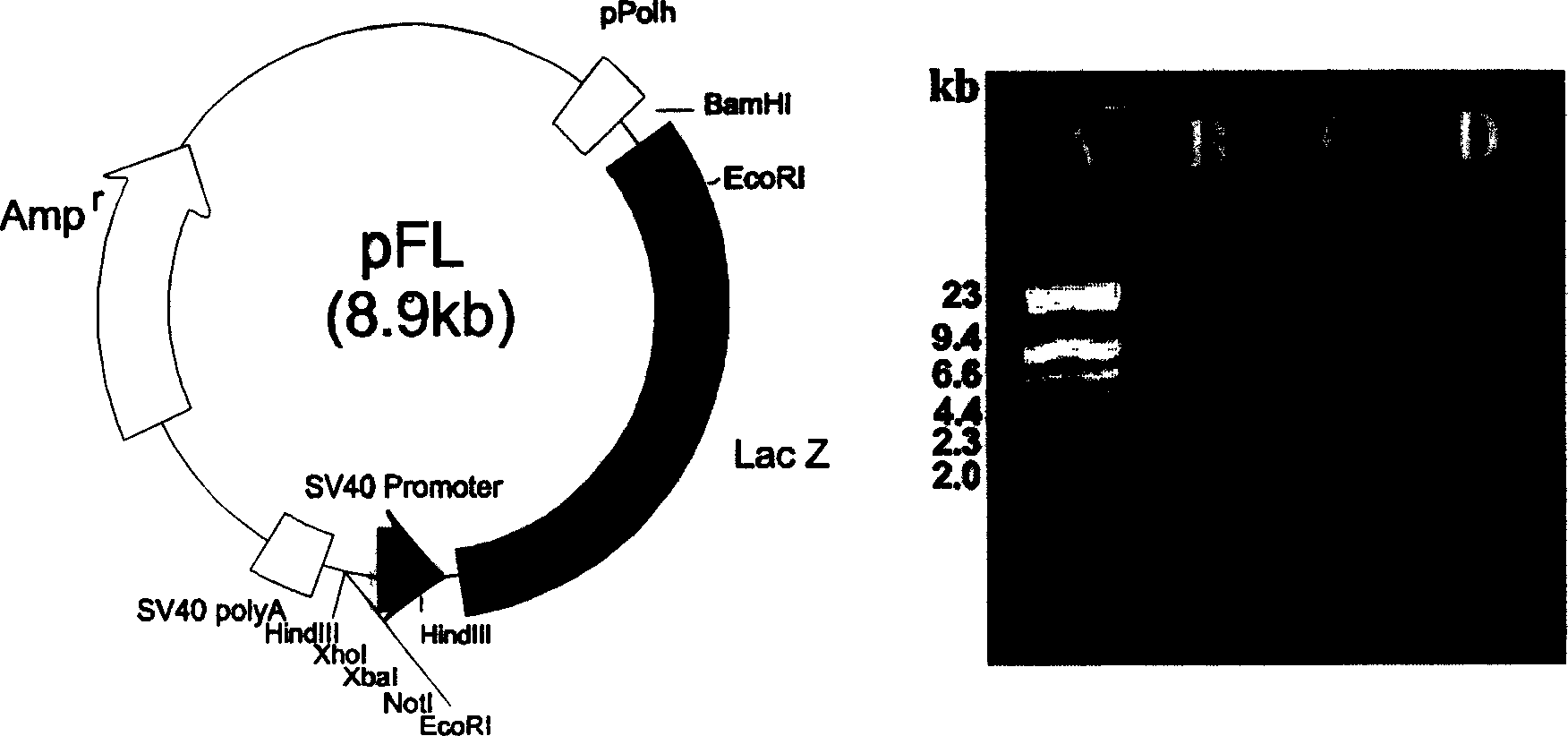
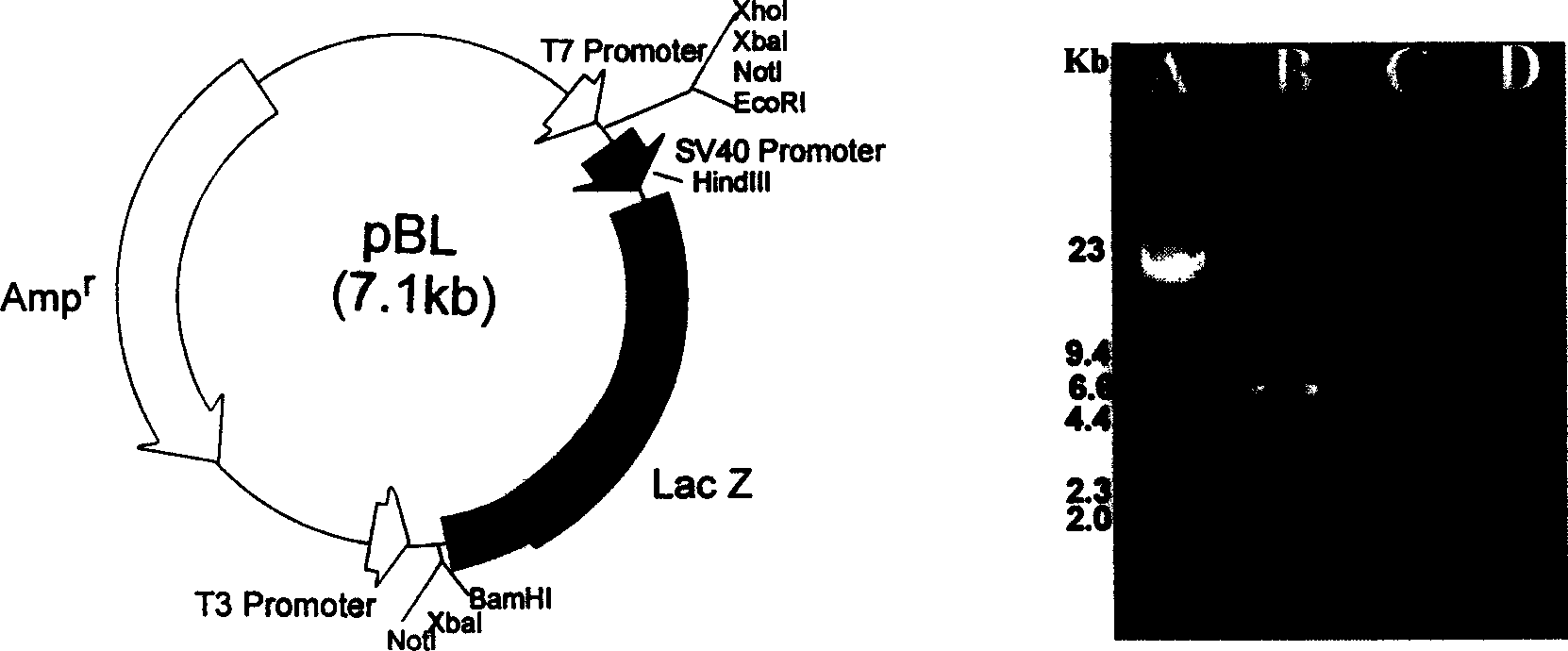
[0056] Construction of the intermediate vector IpFL: from the vector pSV-β-Galactosidase containing the lacZ expression cassette, it was completely digested with BamHI, EcoRI was incompletely di...
Embodiment 2
[0061] Example 2 Identification of recombinant virus rHSV-1Δ34.5 / lacZ
[0062] The tested viruses were the recombinant virus rHSV-1Δ34.5 / lacZ constructed in Example 1 and the wild virus HSV-1 F strain donated by Professor B. Roizman of the University of Chicago. icp34.5 gene primers were synthesized by Shanghai Sangong (SANGON) Company.
[0063] Forward primer: 5'-CTCTGCAGTCACGCCCCTTCCGCCTTCC-3'
[0064] Reverse primer: 5'-CCGGATCCGCTCCTGCCATCGTCTCTCC-3'
[0065] PCR technology was used to amplify icp34.5 gene for identification. Reaction system: 10×buffer 5μL, dNTP (2.5mM) 5μL, forward primer (10pmol / ul) 2.5μL, reverse primer (10pmol / ul) 2.5μL, HSV DNA 5μL, MgCl 2 (25mM) 5μL, ddH 2 O 25 μL, Taq DNA polymerase (5U / ul) 0.5 μL. Amplification conditions: 94°C, 90 seconds; 56°C, 90 seconds; 72°C, 120 seconds; cycle 35 times. Pre-denaturation for 5 minutes (94°C), and final extension for 10 minutes (72°C). The results proved that the recombinant virus rHSV-1Δ34.5 / lacZ delete...
Embodiment 3
[0066] Example 3 Amplification of recombinant virus rHSV-1Δ34.5 / lacZ
[0067] The test virus is the recombinant virus rHSV-1Δ34.5 / lacZ constructed in Example 1. The African green monkey kidney Vero cell line was donated by Professor Dong Changyuan from Wuhan University School of Medicine.
[0068]Use DMEM culture medium to culture Vero cells on a 35mm culture plate. After the Vero cells grow into a single layer, discard the DMEM culture medium, wash the cell surface with PBS, inoculate 0.2ml of virus, absorb at 37°C for 1-2 hours, and discard the upper layer. At the same time, set a bottle of cells without virus as a control; add 2% calf serum medium, and culture at 37°C; after 80% of the cells have lesions, freeze and thaw three times to harvest the virus; centrifuge at 8000rpm at 4°C After 30 minutes, collect the supernatant and store at 4°C for short-term storage; if long-term storage is required, add 10% glycerol and store at -80°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com