Preparation method for resolving optical active cyan alcohol by enzyme process
An optically active and enzymatic separation technology, applied in fermentation and other directions, can solve the problems of low enantiomeric purity, low solubility of cyanohydrin, unfavorable for large-scale production, etc., and achieve high optical purity and improve stability. and optical purity, the effect of large industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
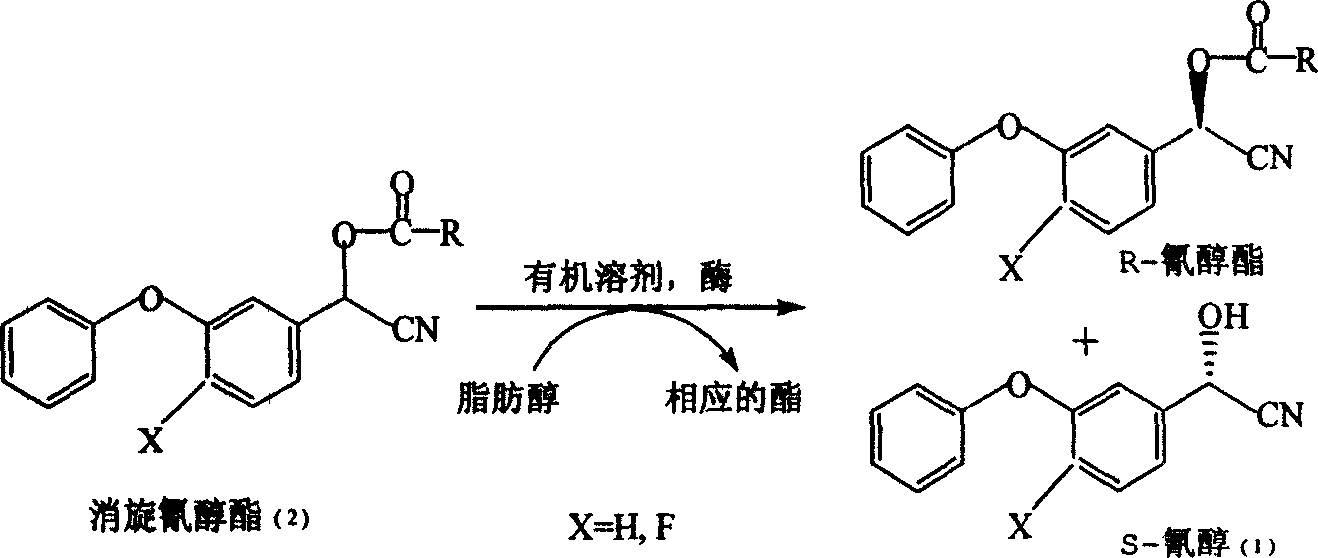
[0016] Example 1: Preparation of S-α-cyano-3-phenoxybenzyl alcohol by enzymatic resolution
[0017] the batch
[0018] The enzyme used in the present invention can be the crude enzyme obtained through preliminary separation and purification after microbial culture; it can also be a commercial enzyme, such as derived from Candida rugosa, Candida cylindracea, Porcine pancreatic, Pseudomonas sp. (Sigma company), Rhizopus delemar, Chromobacterium viscosum, Rhizopus niveus, Aspergillus niger, Aspergillus oryzae, Candida Antarctica, Candida cylindracea, Candida lipolytica, Candida utilis, Mucor javanicus, Rhizopus miehei (Fluka), Alcheigenes sp., Pseudomonas stutzeri (Meito Sangyo, Roarrhizop) and Rhizopus ) and other pure or immobilized enzymes with high selective catalytic ability.
Embodiment 2
[0019] Example 2: Preparation of S-α-cyano-3-phenoxybenzyl alcohol by enzymatic resolution
[0020]Add 0.01mol (2.67g) α-cyano-3-phenoxybenzyl alcohol acetate, 0.02mol n-propanol (1.2g) and 83ml tetrahydrofuran containing 0%~1% water to 250ml at 55°C In the reaction bottle, then add 250mg of enzyme, stir and react for 20 hours. The content of S-α-cyano-3-phenoxybenzyl alcohol and R-α-cyano-3-phenoxybenzyl alcohol in the solution was analyzed by high performance liquid chromatography, and S-α-cyano-3-phenoxy The yield of benzyl alcohol was 49%, and the e.e. value of S-α-cyano-3-phenoxybenzyl alcohol was 99%.
Embodiment 3
[0021] Example 3: Preparation of S-α-cyano-3-phenoxybenzyl alcohol by enzymatic resolution
[0022] Add 0.03mol (7.6g) of α-cyano-3-phenoxybenzyl alcohol formate, 0.12mol of isopropanol (7.2g) and 250ml of ether containing 0% to 1% water to 500ml at room temperature In the reaction bottle, then add 750mg of enzyme, stir and react for 20 hours. The content of S-α-cyano-3-phenoxybenzyl alcohol and R-α-cyano-3-phenoxybenzyl alcohol in the solution was analyzed by high performance liquid chromatography, and S-α-cyano-3-phenoxy The yield of benzyl alcohol was 49.5%, and the e.e. value of S-α-cyano-3-phenoxybenzyl alcohol was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com