Ferrum carried spherical cellulose adsorbent, preparation and application thereof
A cellulose and adsorbent technology, which is applied in the field of iron-loaded spherical cellulose adsorbents, can solve the problems of ineffective removal of arsenite ions, increased operating procedures and costs, and low removal efficiency, and achieves good application prospects and good regeneration. effect, the effect of satisfying the adsorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 16.0g of absorbent cotton (Henan Jiaozuo Hygienic Material Factory) was soaked in 400ml of 20% (m / v) NaOH aqueous solution for 2h, squeezed to about 75g (squeezing ratio is 4:1), to obtain alkali cellulose, the alkali fiber The element was sealed and aged at room temperature for 2-3 days, and 8ml CS was added 2 (CS 2 Milliliters=cotton cellulose dry weight (grams) / 2), seal, shake at 150rpm at room temperature for 4-8 hours to obtain orange-red viscose, add 100ml of 6% NaOH aqueous solution, stir for 3-5 hours to obtain uniform esterification Viscose. The viscose cannot be placed for a long time. Add 200ml of pump oil-chlorobenzene dispersion medium (pump oil: chlorobenzene = 2:1), 0.4g of potassium oleate, 50ml of the above-mentioned viscose liquid into a 500ml three-necked bottle, stir at 200-250rpm for 30 minutes, and then stir for 30-60 Slowly raise the temperature to 90°C within 1 minute, keep it warm for 2 hours, stop stirring, pour out the dispersion medium (th...
Embodiment 2
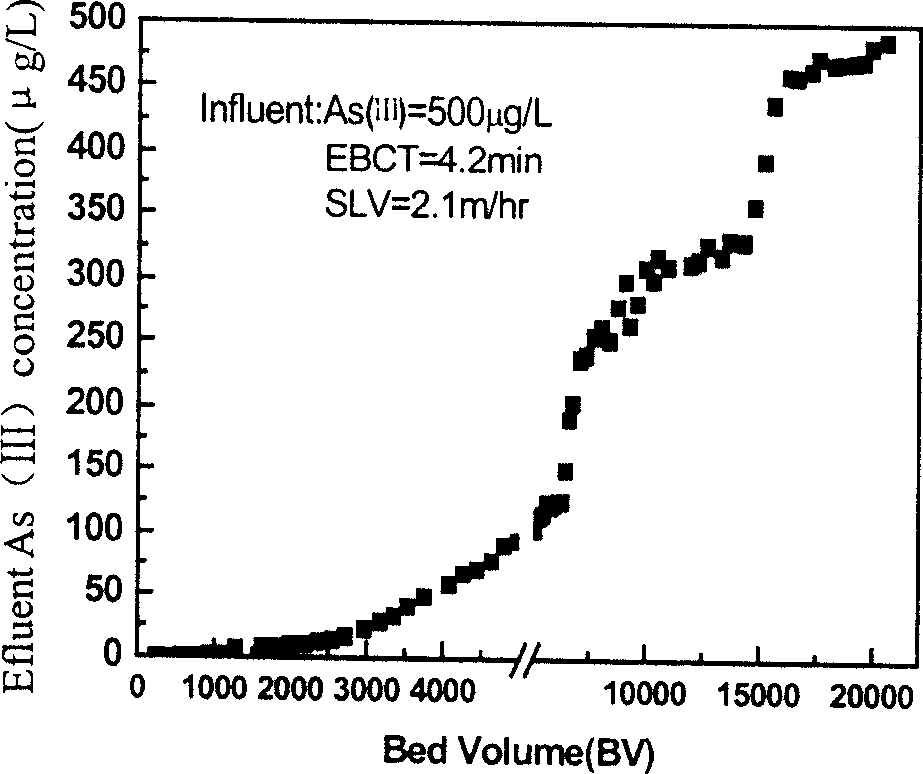
[0030] Take 50ml cellulose beads, add 100ml 5% (m / v) FeCl 3 ·6H 2 O solution, under the stirring condition of 180rpm at room temperature, slowly drop 100-110ml 0.5mol / L NaOH aqueous solution at the injection rate of 12ml / min. When the pH rises to 3.5-4.0, the unloaded part of iron precipitates out, pour out the suspension and the residual ferric hydroxide flocculent precipitate, and wash the beads with water for 5 times. The above-mentioned iron-loading process was repeated 12 times to obtain an iron-loaded spherical cellulose adsorbent with an iron content of 200±20 mg per milliliter, and a mass content of 42% (dry weight).
Embodiment 3
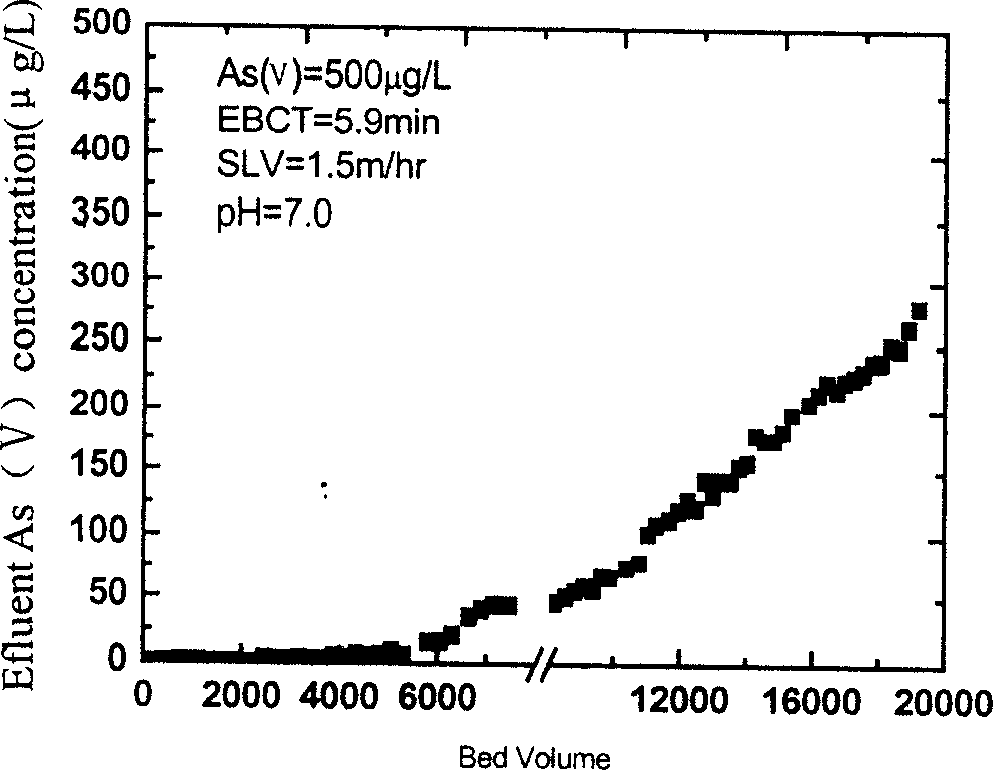
[0032] Take 50ml cellulose beads, add 100ml 10% (m / v) FeCl 3 ·6H 2O solution, under the stirring condition of 180rpm at room temperature, slowly drop into 100-110ml 1.0mol / L NaOH aqueous solution at 10ml / min. When the pH rises to 3.5-4.0, pour out the suspension and the residual ferric hydroxide precipitate, and wash the beads with water for 5 times. The above-mentioned iron-loading process was repeated 7 times to obtain an iron-loaded spherical cellulose adsorbent with an iron content of 200±20 mg per milliliter, and a mass content of 42%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com