Medicine for treating osteomyelitis and preparation thereof
An osteomyelitis and drug technology, which is applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve problems such as few experimental research reports, and achieves simple administration method, low blood concentration, and simple production technology. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Solvent suspension volatilization method to prepare polysebacic anhydride, polylactic acid blended ofloxacin sustained-release tablets
[0038] There are two main methods of preparing polymer material sustained-release tablets: melting-dispersion method and solvent evaporation method (Gao Bo, Luge, Su Tongfang. A biodegradable drug controlled release system implanted into the brain for the treatment of brain tumors[J].Pharmaceutical Progress .2000;24:274). The melting and dispersing method is to heat the polymer to the melting point to make it appear in a molten state, add solid powdered drug according to the required drug loading ratio and stir well, then pour it into a preheated mold, cool it at room temperature, and process it into the required product. size and shape. The solvent evaporation method is to dissolve the polymer material and the loading drug in a certain co-soluble solvent, pour it into the mold at a low temperature, and then further process ...
Embodiment 2
[0048] Example 2 In vitro drug release experiment of polysebacic anhydride, polylactic acid blended ofloxacin sustained-release tablets
[0049] Sampling: Take 3 tablets of polysebacic anhydride and polylactic acid with ratios of 5:95, 10:90, 15:85, 20:80, 25:75 and 30:70 w / w, respectively, and place them in test tubes 10ml of 0.1M phosphate buffer solution (pH7.4) was added to each solution, and the solution was kept in a constant temperature water bath at 37°C. The phosphate buffer solution was replaced at 2 hours, 5 hours, and 10 hours after the experiment started; the phosphate buffer solution was replaced once a day from the 1st to 14th day of the experiment, and then every 2 days from the 15th to 28th day; Phosphate buffer was changed every 3 days for 58 days, and then every 7 days until 3 months after release. After the liquid was taken out and centrifuged, the supernatant liquid was taken and sealed in a test tube, and stored at -20°C until testing.
[0050] Preparat...
Embodiment 3
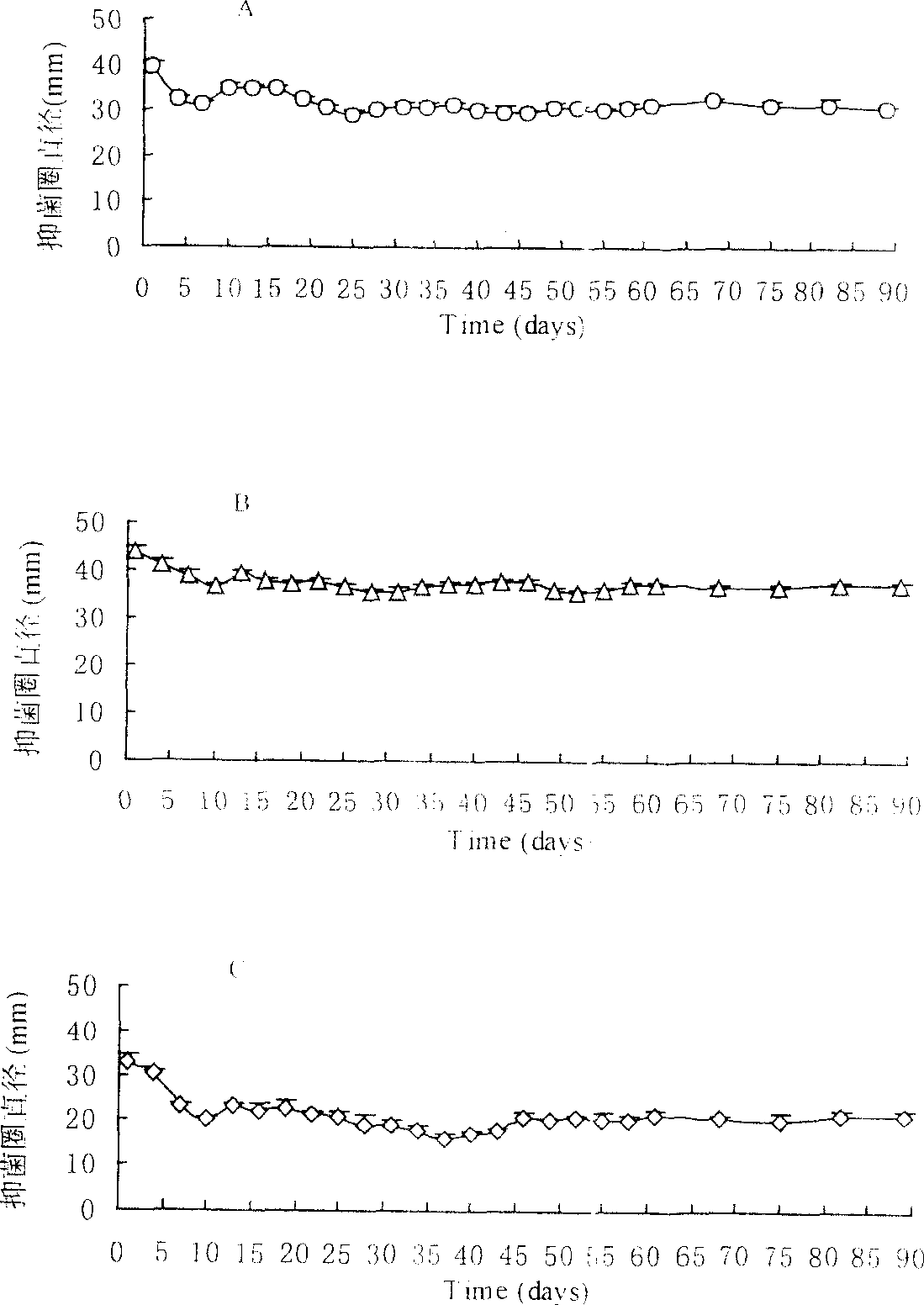
[0057] Example 3 In vitro bacteriostatic experiment of polysebacic anhydride, polylactic acid blended ofloxacin sustained-release tablets
[0058] Take 9 tablets of ofloxacin sustained-release tablets blended with polysebacic anhydride and polylactic acid, each 3 tablets as a group, and place them in the agarose inoculated with Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa respectively. In the center of the culture medium (only one piece is placed in a petri dish), measure the size of the inhibition zone after culturing at 37°C for 24 hours. Then transfer the tablet into a new medium to measure the inhibition zone every 3 days, and replace the new medium, repeating this until 2 months after the experiment, and then replace the new medium every week until 3 months after the experiment. Another tablet of polysebacic anhydride and polylactic acid blend without ofloxacin and of the same size, shape and blending ratio was placed in the above bacterial culture ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com