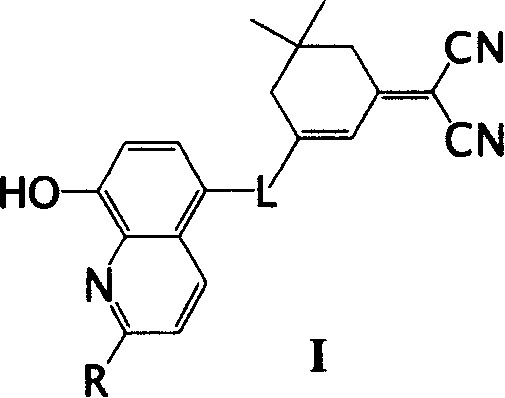
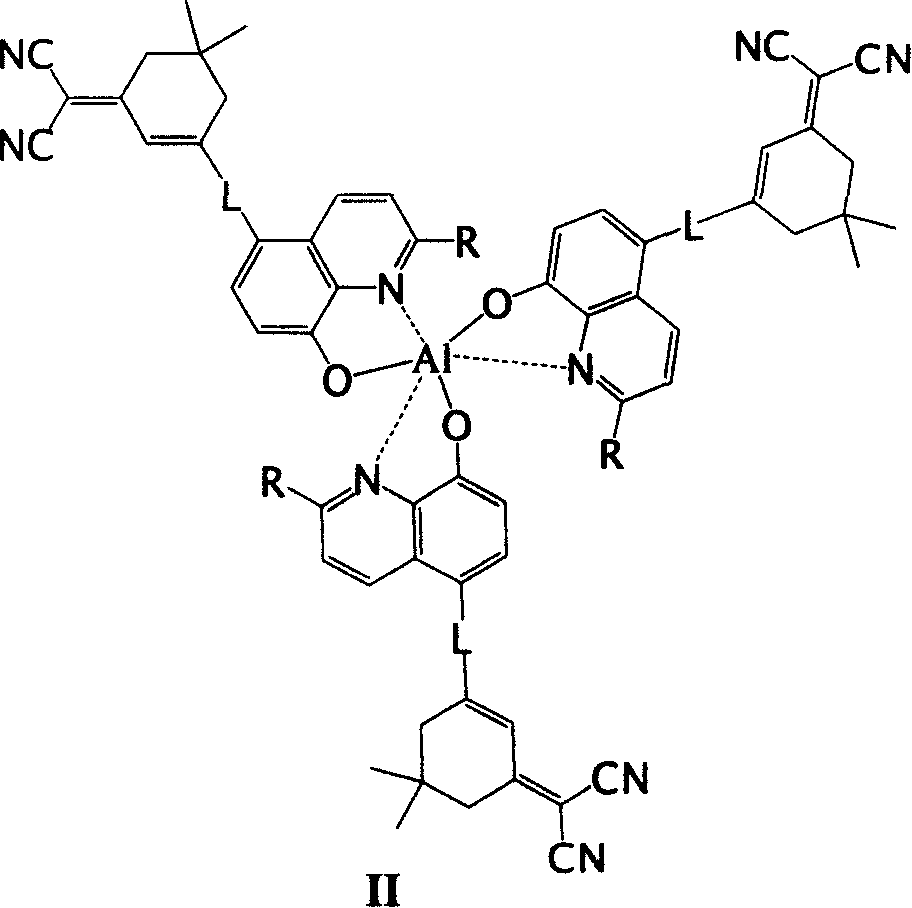
Derivative of 8-hydroxyquinoline of emitting red light
A technology of hydroxyquinoline and derivatives, which is applied in the field of 8-hydroxyquinoline derivatives containing dicyanomethylisophorone, which can solve the problems that the luminous performance of the device is greatly affected, and the luminous chromaticity and brightness are difficult to balance. , to achieve the effect of improving electron transport performance and reducing LUMO orbital
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of compound I-1:
[0022]
[0023] In the flask, add 0.346 gram of 5-formyl-8-hydroxyquinoline (2mmol), 0.372 gram of 2-(3,5,5-trimethylcyclohexene-2-ylidene) malononitrile (2mmol) , 5ml of freshly treated anhydrous acetonitrile and 2 drops of piperidine. Under the protection of argon, the reaction mixture was refluxed for 20h. After the reactant was cooled to room temperature, a dark red precipitate precipitated, which was filtered and washed with 10 ml of acetonitrile. After drying, 0.612 g of dark red powder was obtained, with a yield of 92.8%.
[0024] m.p.253~255℃.
[0025] 1 H NMR (500MHz, CDCl 3 , ppm): δ8.91(d, J=3.5Hz, 1H), 8.66(d, J=7.5Hz, 1H), 7.91(d, J=6.2Hz, 1H), 7.68(s, 1H), 7.65 (t, J=5.5Hz, 1H), 7.40(m, 1H), 7.05(d, 1H), 6.9(s, 1H), 2.64(s, 2H), 2.57(s, 2H), 1.13(s, 6H).
[0026] The maximum absorption wavelength in tetrahydrofuran is 437nm (logε=4.25), the maximum emission wavelength is 603nm, and it is orange-red fluorescence.
Embodiment 2
[0028] Synthesis of Compound 1-2:
[0029]
[0030] (1) Synthesis of (E)-2-(3-(4-nitrostyryl)-5,5-dimethylcyclohex-2-enyl) malononitrile (compound A):
[0031] In the flask, add 0.66 g of p-nitrobenzaldehyde (4.4 mmol), 0.81 g of 2-(3,5,5-trimethylcyclohexene-2-ylidene) malononitrile (4.4 mmol), 5 ml of new Treat with anhydrous acetonitrile and 2 drops of piperidine. Under the protection of argon, it was refluxed for 2h. After the reactant was cooled to room temperature, a tan precipitate precipitated, which was filtered and washed with 10 ml of acetonitrile. After drying, 1.05 g of brown yellow powder of compound A was obtained, with a yield of 72.0%.
[0032] (2) Synthesis of (E)-2-(3-(4-aminostyryl)-5,5-dimethylcyclohex-2-enyl)malononitrile (compound B):
[0033] In a flask, 1.05 g of compound A (3.3 mmol), 3.70 g of stannous dichloride dihydrate (16.4 mmol), 20 ml of ethanol and 2 drops of glacial acetic acid were added. Under the protection of argon, it was reflux...
Embodiment 3
[0040] Synthesis of Compound I-3
[0041]
[0042]
[0043] (1) References for the preparation of 8-hydroxy-2-methylquinoline-5-sulfonyl chloride (J.A.C.S.2001, 123, 5160), references for the synthesis of p-piperazinyl benzaldehyde (Dyes and Pigments 2003, 59, 143 ).
[0044] (2) Synthesis of Compound C
[0045] In the flask, add 0.19 g of p-piperazinyl benzaldehyde (1.0 mmol), 0.20 g of potassium carbonate (1.5 mmol) and 20 ml of dichloromethane, after stirring for 20 min, add 0.26 g of 8-hydroxyl-2-methylquine in batches Phenyl-5-sulfonyl chloride (1.0 mmol), stirred at room temperature for 4h. Pour into 50ml of water, extract with dichloromethane, and dry the organic phase with anhydrous sodium sulfate. After rotary evaporation, a tan powder was obtained, which was passed through a silica gel column with a developing solvent of chloroform / methanol (4:1). Collect the first component, 0.32 g of light-colored powder after rotary evaporation. Yield 77.8%.
[0046] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com