Mono-methoxy polyethylene glycol derivatives and their preparing process and use
A technology of polyethylene glycol silane and monomethoxy, which is applied in biological testing, silicon organic compounds, chemical instruments and methods, etc., can solve the problems of complex processing process and weak inhibitory effect, and achieve simple processing process and inhibitory effect. Effect of non-specific adsorption and mild use conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
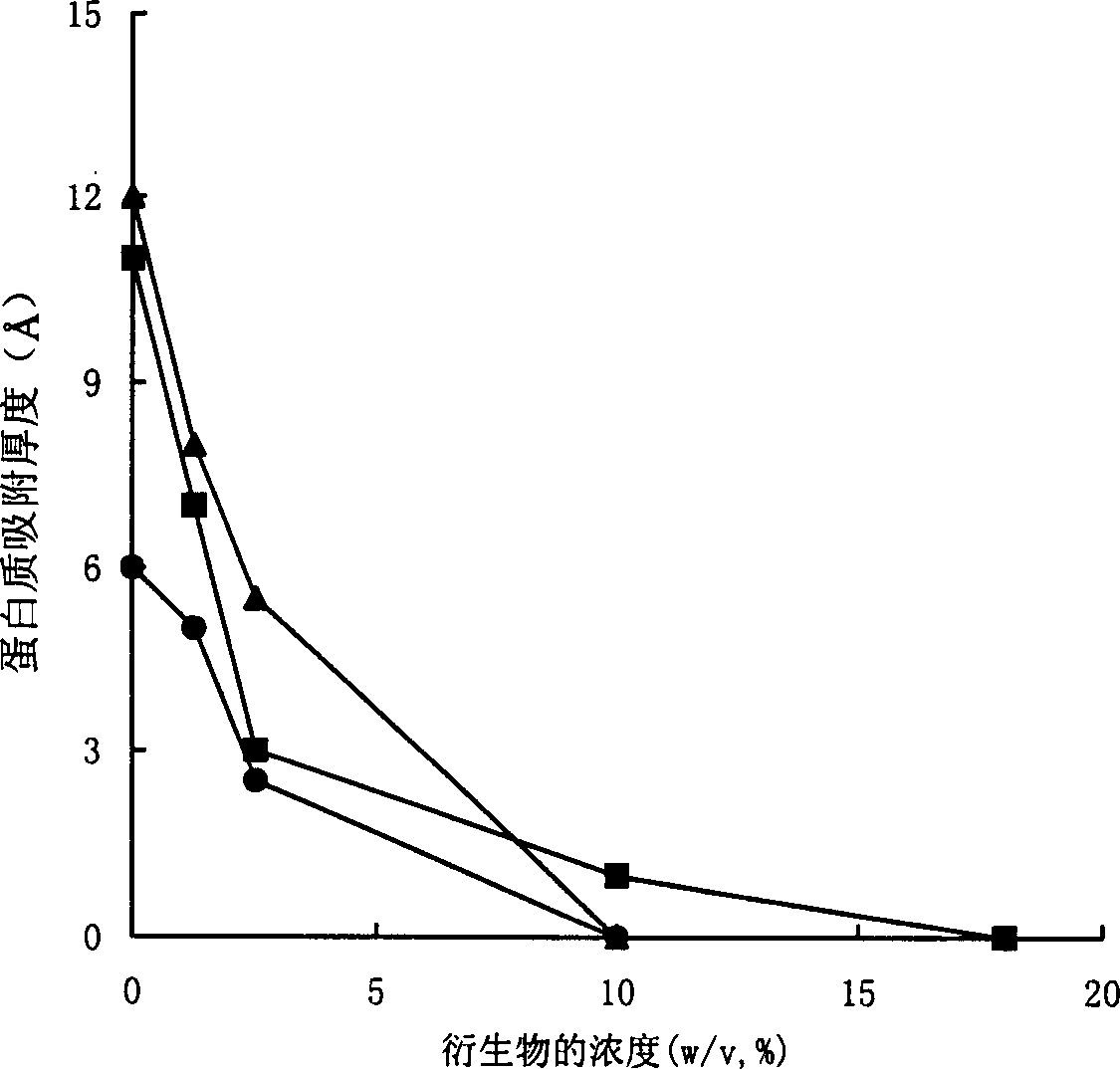
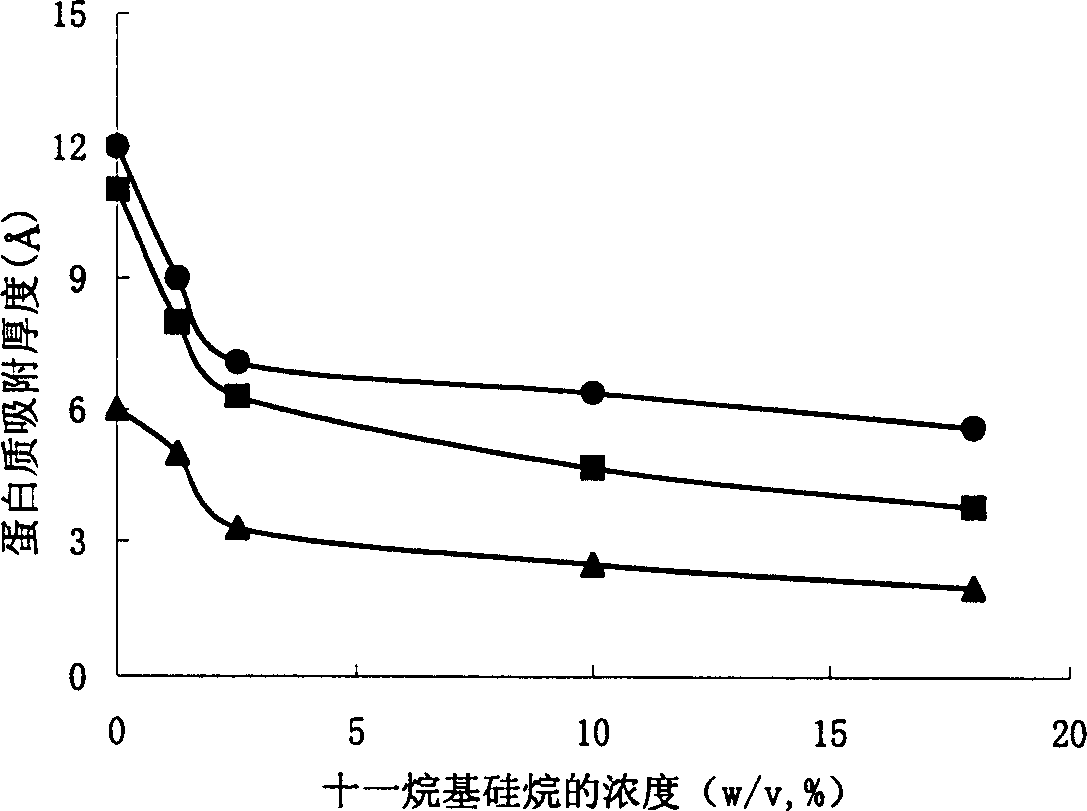
Examples
Embodiment 1
[0030] Embodiment 1, preparation monomethoxy polyethylene glycol silane derivative 1
[0031] Dissolve monomethoxytriethylene glycol (CH 3 O(CH 2 CH 2 O) 3 H) 6.560 g (40 mmol) and 4.444 g (44 mmol) of triethylamine, cooled in an ice-salt bath. Another 8.426g (44mmol) of p-toluenesulfonyl chloride was dissolved in 50ml of dichloromethane. Under electromagnetic stirring, the p-toluenesulfonyl chloride-dichloromethane solution was added dropwise to the reaction solution. Afterwards, the ice-salt bath was removed, returned to room temperature, and continued to stir for 24 hours. Filtration, the filtrate was concentrated by rotary evaporation, and then petroleum ether: ethyl acetate = 1:1 was used as the eluent, and silica gel column chromatography was used to separate to obtain 9.540 g (30 mmol) of monomethoxytriethylene glycol p-toluenesulfonate. Yield 75%.
[0032] 897 mg (39 mmol) of sodium metal was dissolved in 6 ml of methanol to obtain 39 mmol of sodium methoxide. ...
Embodiment 2
[0033] Embodiment 2, preparation monomethoxy polyethylene glycol silane derivative 2
[0034] The same method as in Example 1 was used.
[0035] Dissolve 1.748g (23mmol) monomethoxyethylene glycol (CH 3 OCH 2 CH 2 OH) and 2.362g (30mmol) of pyridine, cooled in an ice-salt bath. Another 5.745g (30mmol) of p-toluenesulfonyl chloride was dissolved in 50ml of dichloromethane. Under electromagnetic stirring, the p-toluenesulfonyl chloride-dichloromethane solution was added dropwise to the reaction solution. After that, the ice-salt bath was removed, returned to room temperature, and stirred for 24 hours. Filtration, the filtrate was concentrated by rotary evaporation, and then using petroleum ether: ethyl acetate = 1:1 as eluent, silica gel column chromatography to obtain 3.45g (15mmol) monomethoxyethylene glycol p-toluenesulfonate, Yield 65.2%.
[0036]345 mg (15 mmol) of sodium metal was dissolved in 4 ml of methanol to obtain 15 mmol of sodium methoxide. After that, 2.7 ...
Embodiment 3
[0037] Embodiment 3, preparation monomethoxy polyethylene glycol silane derivative 3
[0038] The same method as in Example 1 was used.
[0039] Dissolve 1.92g (5mmol) monomethoxy octaethylene glycol (CH 3 O(CH 2 CH 2 O) 8 H) and 5.925g (7.5mmol) of pyridine, cooled in an ice-salt bath. Another 1.436g (7.5mmol) p-toluenesulfonyl chloride was dissolved in 50ml of dichloromethane. Under electromagnetic stirring, the p-toluenesulfonyl chloride-dichloromethane solution was added dropwise to the reaction solution. Afterwards, the ice-salt bath was removed, returned to room temperature, and continued to stir for 24 hours. Filtration, the filtrate was concentrated by rotary evaporation, and then petroleum ether: ethyl acetate = 1:1 was used as the eluent, separated by silica gel column chromatography to obtain 1.668 g (3.1 mmol) of monomethoxy octaethylene glycol p-toluenesulfonate , yield 62%.
[0040] 78 mg (3.4 mmol) of sodium metal was dissolved in 4 ml of ethanol to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com