Organic pore-borne material generated by self-assembly of organic and organic, and preparation method
A mesoporous material and self-assembly technology, applied in the field of polymer materials, can solve the problems of material order, low specific surface area and pore volume, no covalent bond network structure, complex synthesis methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of polymer precursor:
[0041] The molar ratio of reaction material: phenol (cresol, xylenol): formaldehyde (furfural)=1: (0.8-2.1)
[0042] Reaction temperature: 65-70°C; Reaction time: 0.5-2 hours;
[0043] PH=1-3 or 7-11
[0044] Acidic catalysts: chlorosulfonic acid, hydrochloric acid, perchloric acid, sulfuric acid, oxalic acid
[0045] Basic catalyst: NH 3 ·H 2 O, NaOH, KOH, Ba(OH) 2 ;
[0046] The example scheme is as follows:
[0047] Melt 1.0g phenol at 40-42°C, add 0.21g 20% NaOH aqueous solution at this temperature and stir for ten minutes, add 1.7g 37% formaldehyde aqueous solution, heat up to 70°C for 1 hour, cool to room temperature, and use 0.6M HCl The solution adjusts the pH value of the solution to 6-7, dehydrating under reduced pressure at a temperature lower than 50°C.
Embodiment 2
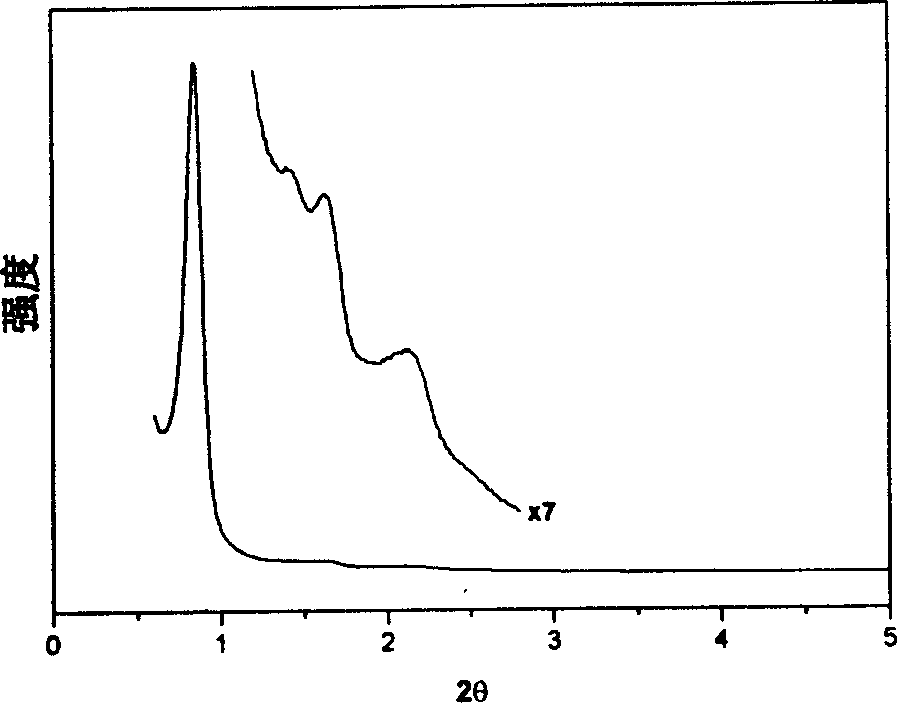
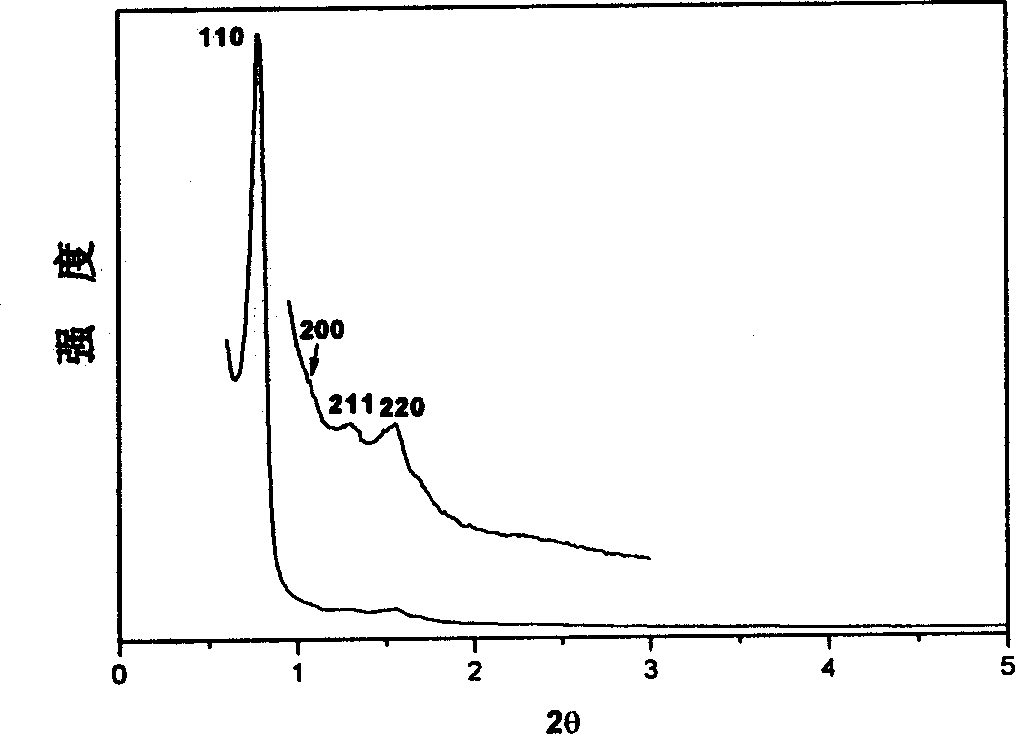
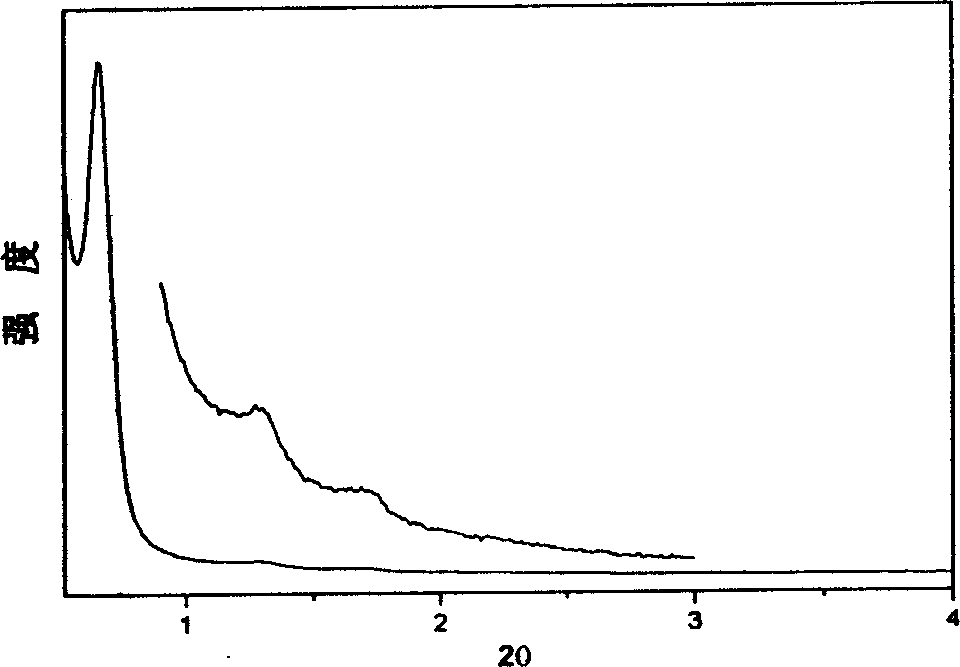
[0048] Example 2, at room temperature (25°C), 1.0g EO 106 PO 70 EO 106 Dissolve in 20g of ethanol, stir to obtain a clear solution, add phenolic resin polymer prepolymer (prepared from 0.61g phenol and 0.39g formaldehyde) to this solution, stir for 5 minutes, make it evenly dispersed, and form a uniform mixture. Transfer the solution to a watch glass, let it stand at 25°C to volatilize the ethanol, and after 8 hours, the ethanol is completely volatilized, and then placed in an oven at 100°C for 24 hours to further polymerize the polymer material. The polymerized material was calcined at 350° C. for 5 hours under a nitrogen atmosphere to remove the surfactant, and the polymer mesoporous material was obtained. The material has a pore diameter of 6.8nm and a pore volume of 0.63cm 3 / g, the specific surface area is 652m 2 / g, the pore space symmetry of the material is a hexagonal p6m structure.
Embodiment 3
[0049] Example 3, at room temperature (25°C), 1.0g EO 106 PO 70 EO 106 Dissolve in 30g of ethanol, stir to obtain a clear solution, add phenolic resin polymer prepolymer (prepared from 0.61g phenol and 0.39g formaldehyde) to this solution, stir for 5 minutes, make it evenly dispersed, and form a uniform mixture. Transfer the solution to a watch glass, let it stand at 15°C to volatilize the ethanol, and after 8 hours, the ethanol is completely volatilized, and then placed in a 1000 oven for 48 hours to further polymerize the polymer material. The polymerized material was treated with 48% H 2 SO 4 Refluxing at 95°C for 24 hours to extract and remove the surfactant to obtain a polymer mesoporous material. This material has a pore volume of 071cm 3 / g, the specific surface area is 1042m 2 / g, the pore space symmetry of the material is a hexagonal p6m structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com