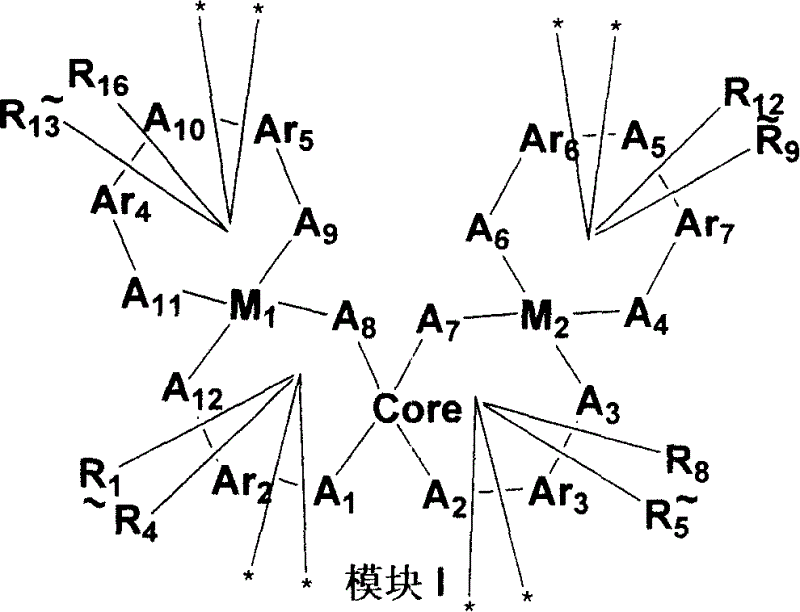
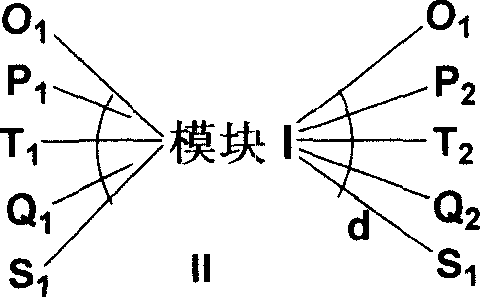
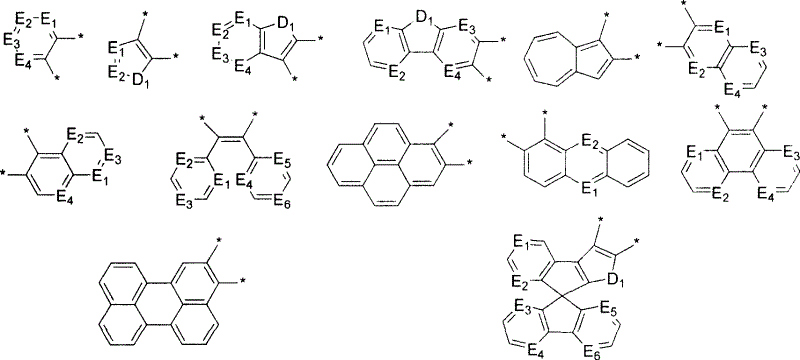
Non-benzenoid aryl spiro material and its synthesis and application
A technology of double spiro ring and meaning, applied in the field of double spiro ring material and preparation thereof, can solve the problems of no report, few articles and patent reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] Example 1. Preparation of all-thiophene double-spiro unit modules:
[0214] 3,4-Dibromo-2,5-bis(trimethylsilyl)thiophene
[0215] 3,4-dibromo-2,5-bis(trimethylsilyl)thiophene
[0216]
[0217] Lithium diisopropylamide (LDA, 2equiv.) was added dropwise to -78°C tetrahydrofuran (0.424mol / L) produced from acetone and dry ice; then 2,5-dibromothiophene (106.75mmol, 1equiv. ) for 45 minutes, then reacted at -78°C for one hour, then returned to room temperature and reacted for another 2 hours, quenched the reaction with ice water, extracted with ether, dried and rotary evaporated, purified with petroleum ether silica gel column, and obtained white crystal 3,4- 4.51 g of dibromo-2,5-bis(trimethyl)thiophene (45% yield). GC-MS (EI-m / z): 386 (M + ); elemental analysis: calculated value C 10 h 18 Br 2 SSi 2 : C, 31.09; H, 4.69; S, 8.30. Measured values: C, 31.15; H, 4.74; S, 8.33.; 1 H NMR (400MHz, CDCl 3 , ppm): 0.38 (s, Me 3 Si); 13 C NMR (50.31MHz, CDCl 3 : TMS) ...
Embodiment 2
[0228] Example 2, preparation of thiophene double spiro unit containing diazafluorene:
[0229]
[0230] Take 2,5-dibromo-3,4-bis(thiophen-3-yl)thiophene (2.1mmol) and magnesium Mg 0.1004g (4.2mmol) to react to generate Grignard reagent, dissolve in 16mL tetrahydrofuran with 4,5- Diazofenone (4.2mmol) was reacted at 60°C for 12 hours, a large amount of white precipitate was generated, and finally saturated color NHCl was added 4 Convert Grignard salts to alcohols. After the reaction was completed, extracted with ether, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly yellowish white solid tertiary alcohol (yield 56%). Dissolve the above-mentioned fluorenol in glacial acetic acid, add a few drops of concentrated hydrochloric acid dropwise at 110°C for 10 minutes to form a white precipitate, add water to produce more precipitate, filter, and recrystallize with tetrahydrofuran and...
Embodiment 3
[0231] Example 3, preparation of fluorene-containing thiophene double spiro unit:
[0232]
[0233] Take 2,5-dibromo-3,4-bis(thiophen-3-yl)thiophene (2.1mmol) and magnesium Mg 0.1004g (4.2mmol) to react to generate Grignard reagent, and dissolve fluorenone (4.2mmol) in 16mL tetrahydrofuran mmol) was reacted at 60°C for 12 hours, a large amount of white precipitate was generated, and finally saturated NHCl was added 4 Convert Grignard salts to alcohols. After the reaction was completed, ether was extracted, dried and rotary evaporated, and purified on a silica gel column with a mixed solvent of petroleum ether:dichloromethane (3:2) to obtain a slightly yellowish white solid tertiary alcohol (68% yield). Dissolve the above-mentioned fluorenol in glacial acetic acid, add a few drops of concentrated hydrochloric acid dropwise at 110°C for 10 minutes to form a white precipitate, add water to produce more precipitate, filter, and recrystallize with tetrahydrofuran and petroleum ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com