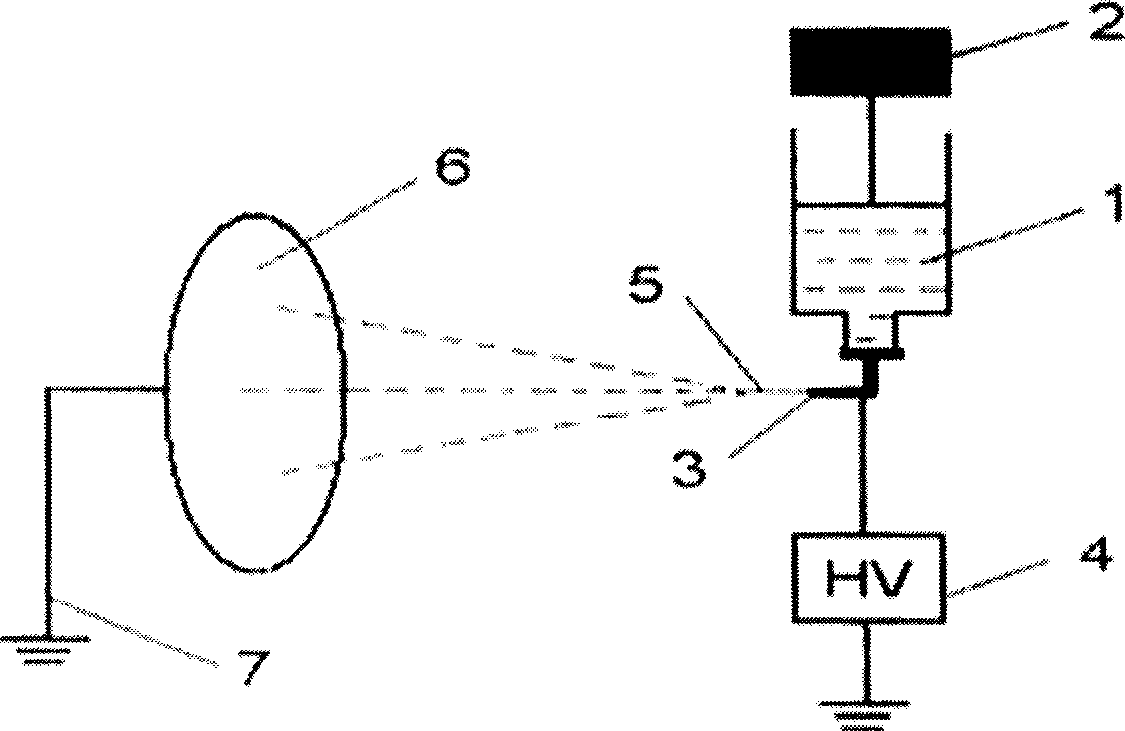
Preparation method of ultrafine fiber medical agent type emulsion electro spinning fiber
A technology for pharmaceutical dosage forms and ultra-fine fibers, which can be applied in fiber processing, rayon manufacturing, fiber chemical characteristics, etc., can solve the problems of unfavorable controlled drug release, no phase separation, low thermal stability, etc., and achieves short cycle time and cost. low, high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (a) Dissolve 0.058 g of doxorubicin hydrochloride (2.46% of the polymer weight) in 1.67 ml of distilled water;
[0056] (b) Dissolve 2.3617 g of PLLA-PEG (the number average molecular weight of the copolymer is 84800 and the number average molecular weight of the PEG segment is 750) in 25 ml of chloroform to obtain a transparent and uniform solution. Then 0.1181 g of sodium lauryl sulfate was added to the solution as a surfactant. Since sodium lauryl sulfate is insoluble in chloroform, it can be uniformly dispersed in the chloroform solution of PLLA-PEG by ultrasonic vibration or mechanical stirring;
[0057] (c) Place the solution obtained in step (b) in a beaker, turn on the high-shear mixing emulsifier for pre-emulsification for 2 minutes, keep the emulsification rate at 7000 rpm, and slowly drop the aqueous solution of adriamycin hydrochloride into the solution in. After the dripping is completed, continue emulsification for 15 minutes to obtain a stable W / O emulsion. D...
Embodiment 2
[0067] (a) Dissolve 0.0401 g of doxorubicin hydrochloride (2.06% of the polymer weight) in 1.67 ml of distilled water;
[0068] (b) Dissolve 1.9474 g of PLLA-PEG (the number average molecular weight of the copolymer is 97600 and the number average molecular weight of the PEG segment is 5000) in 25 ml of chloroform to obtain a transparent and uniform solution. Then 0.0974 g of sodium lauryl sulfate was added to the solution as a surfactant. Since sodium lauryl sulfate is insoluble in chloroform, it can be uniformly dispersed in the chloroform solution of PLLA-PEG by ultrasonic vibration or mechanical stirring;
[0069] (c) Same as step (c) in Example 1, except that the emulsification rate is 6500 rpm;
[0070] (d) Electro-spinning the W / O emulsion obtained in step (c) to obtain an ultrafine fiber pharmaceutical dosage form supporting water-soluble drugs. The parameters in the electrospinning process are: a right-angle flat nozzle made of a No. 7 needle is used, the spinning flow ra...
Embodiment 3
[0072] (a) Dissolve 0.048 g of doxorubicin hydrochloride (3.33% of the polymer weight) in 1.67 ml of distilled water;
[0073] (b) Dissolve 1.1443g PLLA (viscosity average molecular weight of 153,000) and 0.0572g glycerol monostearate in 25ml chloroform;
[0074] (c) Same as step (c) in Example 1;
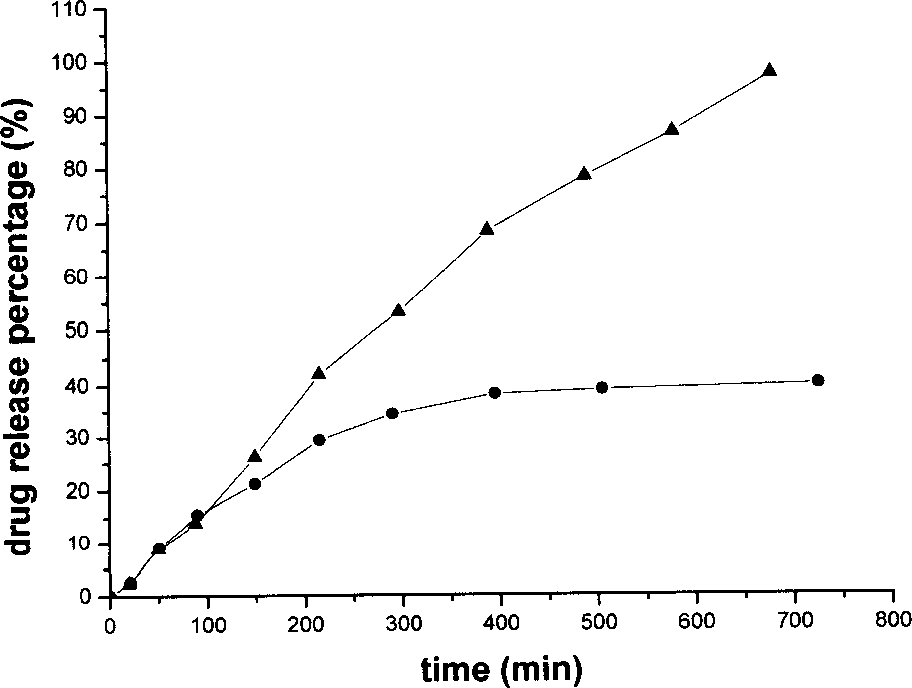
[0075] (d) Electro-spinning the W / O emulsion obtained in step (c) to obtain an ultrafine fiber pharmaceutical dosage form supporting water-soluble drugs. The parameters in the electrospinning process are: a right-angle flat nozzle made of a No. 7 needle is used, the spinning flow rate is 3.5ml / h, the applied voltage is 35kV, and the distance between the two poles is 18cm. The average diameter of the obtained drug-loaded fiber is 600nm, see Figure 7 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com