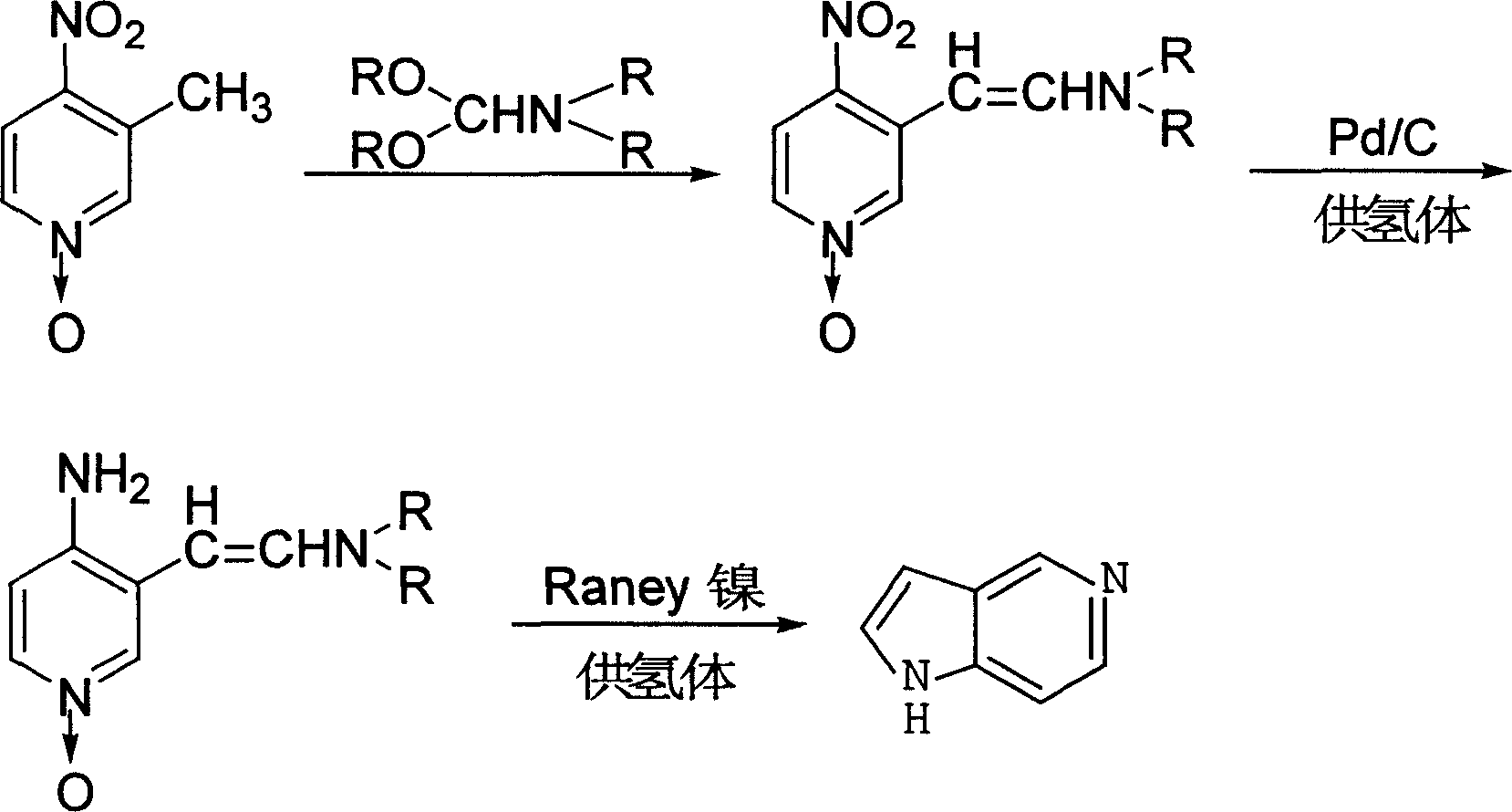
5-aza-indole preparation method
A technology of azaindole and nitropyridine nitrogen oxide, which is applied in the field of preparation of 5-azaindole, can solve the problems of incomplete reaction, lower reaction temperature, incomplete reaction, etc., and achieve rapid and mild reaction, color and luster Good, easy to recycle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The first step: 46.2 grams (0.3mol) of 3-methyl-4-nitropyridine nitrogen oxide, N, N-diethylformamide diethyl acetal 70ml, N, N-dimethylformamide 20ml Put it into a 1L four-neck flask, heat it slowly to 130°C under stirring conditions, react for 1.5 hours, distill off the ethanol generated in the reaction, and then recover the excess acetal and solvent N,N-dimethylacetamide under reduced pressure . The reddish-brown residue was dissolved in boiling ethanol, refrigerated overnight, and the precipitated solid was filtered by suction and dried to obtain purple-red crystal 3-dimethylaminovinyl-4-nitropyridine nitrogen oxide, which was dried and weighed 59.6g, with a yield of 95.1 %.
[0021] Step 2: Put 45g of 3-dimethylaminovinyl-4-nitropyridine nitrogen oxide, 2.7g of 10% palladium carbon, and 300ml of ethanol into a 500ml four-necked flask, cool it to 15°C in an ice bath, and start slowly dripping Add 35ml of 85% hydrazine hydrate, remove the ice bath after dropping, k...
Embodiment 2
[0024] Step 1: Replace N,N-dimethylformamide acetal with 65ml N,N-dimethylformamide acetal, 16ml N,N-dimethylformamide instead of N,N-dimethylformamide Formamide was slowly heated to 100°C under stirring conditions, and the rest were the same as in the first step of Example 1 to obtain purple-red crystal 3-dimethylamine vinyl-4-nitropyridine nitrogen oxide, which weighed 59.3g after drying. Yield 94.6%. ;
[0025] The second and third steps were the same as in Example 1 to obtain 21.8 g of 5-azaindole.
Embodiment 3
[0027] The first step is with embodiment 1;
[0028] Second step: replace hydrazine hydrate with 44g anhydrous ammonium formate, and mother liquor directly carries out next step reaction;
[0029] The third step is the same as in Example 1 to obtain 22.3g of 5-azaindole
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com