Polypeptide gapM1 and its prepn process
A sequence and cell technology, applied in the field of gapM1 and its preparation, and new peptides, can solve the problems of inconvenient production process, high production cost, difficult to control the production process, etc., and achieve the effect of easy production process, low production cost and high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
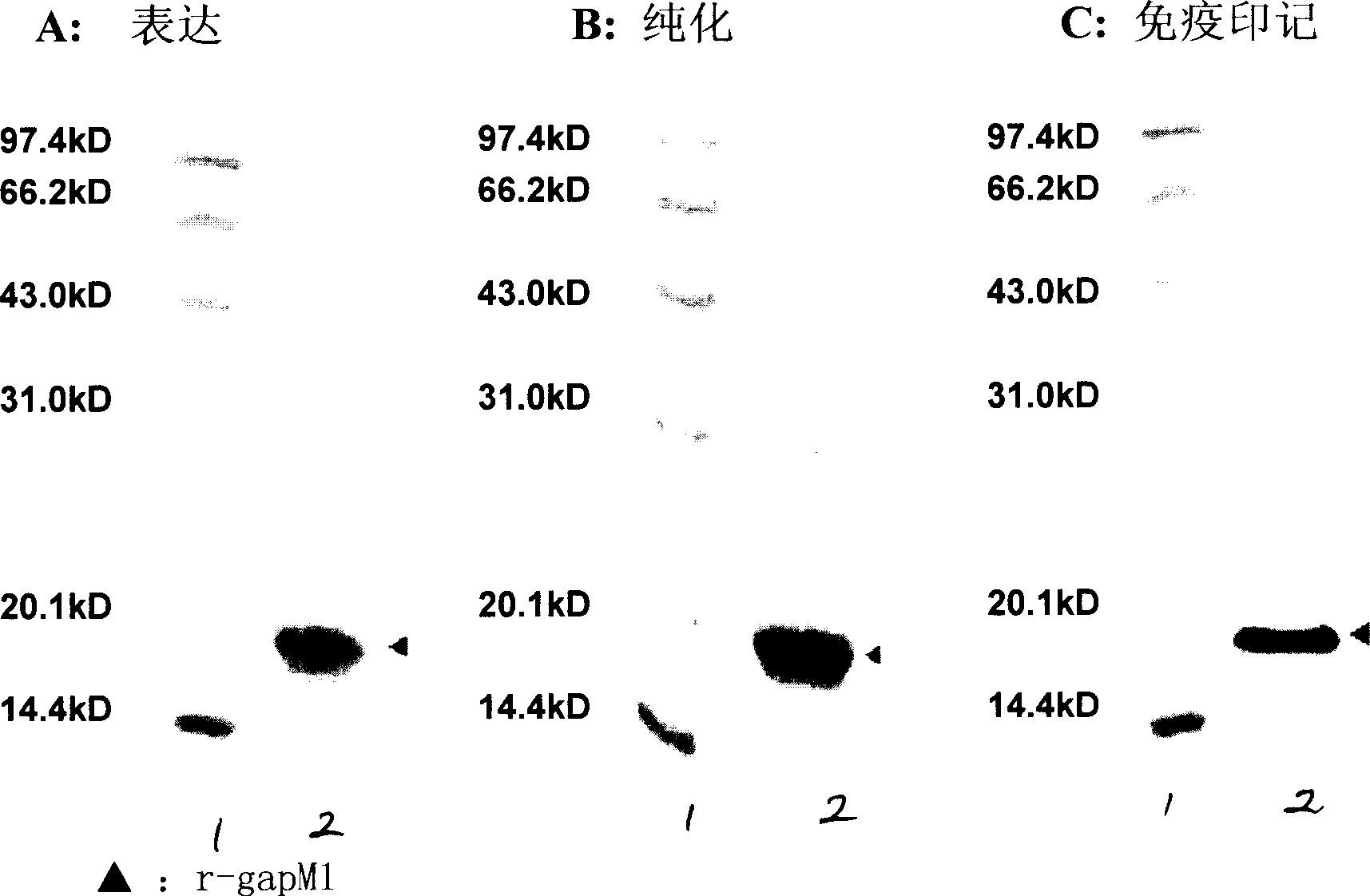
[0031] Example 1 Optimization and preparation of gapM1 gene
[0032] (1) Optimization of gapM1 gene and construction of eukaryotic expression plasmid rpPIC9K / gapM1:
[0033] The optimization method of gapM1 gene is as follows: according to the preference of codon usage of Pichia pastoris, the cDNA of gapM1 is optimized and transformed. The PCR method is used to amplify the optimized coding sequence of gapM1 by using primers and cDNA of wild gapM1 as a template. The optimized gene was recombined with the eukaryotic expression vector pPIC9K, and the nucleotide sequence analysis confirmed that the gene sequence was consistent with the expectation.
[0034] The gapM1 codon optimization method is as follows:
[0035] Using the primer amplification method, according to the preference of yeast codon usage, synthesize primers, and use the cDNA sequence of wild-type gapM1 as a template to carry out PCR. The PCR product is the gene encoding the optimized gapM1. The optimized coding ...
Embodiment 2
[0052] The property determination of embodiment 2 gapM1
[0053] The gapM1 prepared by the present invention is used for activity measurement, and it is confirmed that it has the effect of lowering blood sugar, blood fat, ie body weight.
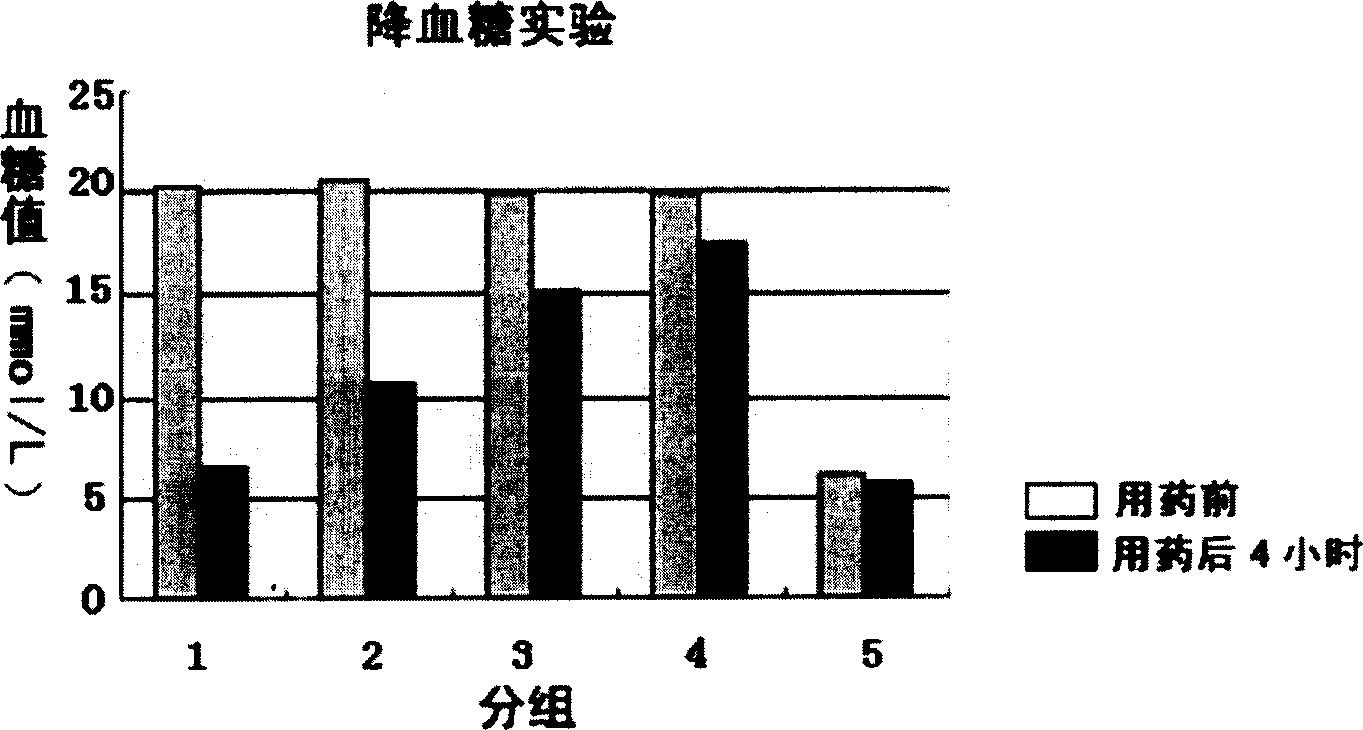
[0054] (1) Determination of hypoglycemic activity,
[0055] Methods: C57BL / 6J mice were intraperitoneally injected with high-dose streptozotocin (STZ) once to create insulin-resistant diabetic models. The control group was given normal saline, the treatment group was given rgapM1, and the fasting blood glucose level of the mice was measured 4 hours later.
[0056] see results figure 2 . The results showed that rgapM1 had the effect of lowering blood sugar significantly;
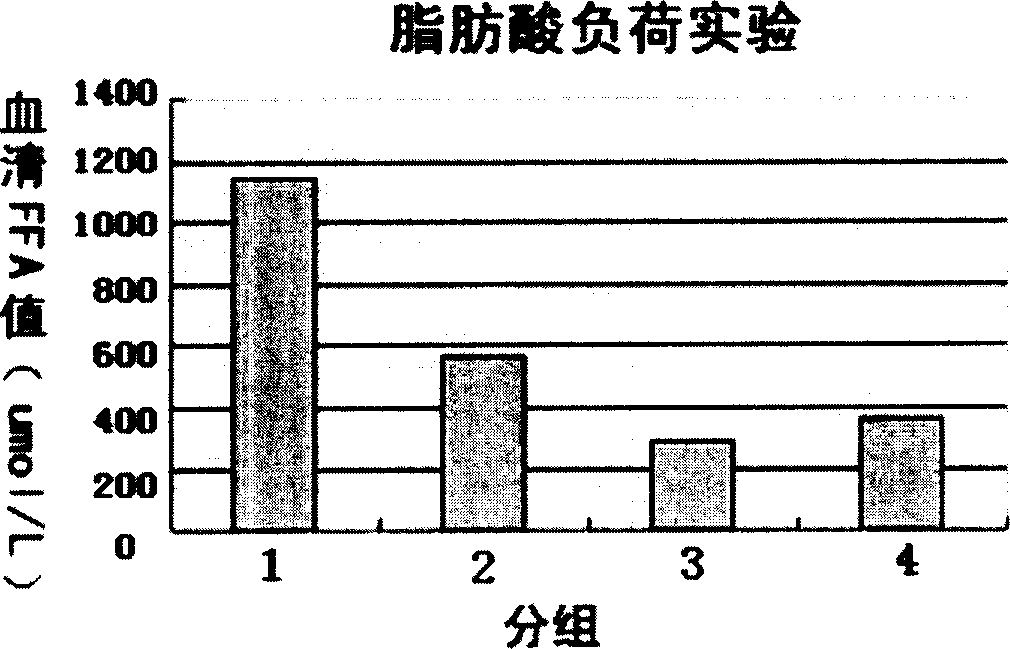
[0057] (2) Determination of blood lipid lowering activity,
[0058] C57BL / 6J mice were injected with a certain dose of fat emulsion into the tail vein to create an acute hyperlipidemia model. Then a certain dose of rgapM1 was injected intraperitoneally one hour later...
Embodiment 3
[0063] Embodiment 3 The inventive method compares with prior art advantage
[0064] The previous production method in the prior art is to express rgapM1 in the Escherichia coli system, and the expressed rgapM1 exists in the form of inclusion body (inactive form). Therefore, a renaturation process is required after rgapM1 is expressed and purified. Due to the characteristics of the spatial structure, it is difficult to renature after denaturation, and the direct result is low production rate and low biological activity. At the same time, due to the complexity of the renaturation process, the protein after renaturation has the problem of uneven activity and the activity cannot fully restore the natural activity.
[0065] The advantage of the method of the present invention is that: using the eukaryotic expression system, the rgapM1 is secreted into the culture medium by the host cell after being synthesized, and exists in the form of a soluble protein with natural activity. At...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com