Process for preparing chlorine blended metal oxide catalyst
A technology of oxide and chlorine doping, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., and can solve the problems of low activity and easy poisoning of metal oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 33.9 mg of ammonium chloride and 0.727 g of cobalt nitrate in a 50 ml beaker, add 25 ml of deionized water to dissolve to obtain a mixed solution, weigh 3.6 g of alumina, soak in the above solution for 6 hours, and filter to obtain a catalyst precursor. The catalyst precursor was dried in an oven at 60° C. for 8 hours, and the moisture was evaporated to obtain a dried catalyst precursor. Then, in the muffle furnace, the temperature was raised to 100° C. at a rate of 5° C. / minute, and kept for 0.5 hours to obtain a chlorine-doped cobalt metal oxide catalyst with high catalytic activity.
[0028] The mercury oxidation performance test of the chlorine-doped cobalt metal oxide catalyst adopts a fixed reaction bed. The fixed reaction bed uses a quartz glass tube with a diameter of 6 mm, and 5 mg of quartz wool is blocked in the middle of the quartz tube. The concentration of mercury in the gas is controlled using a mercury permeation tube. Concentration of elemental m...
Embodiment 2
[0030] Pour 15ml of hydrogen chloride aqueous solution (0.1mol / L) and 5ml of ferric oxalate solution with a concentration of 0.2g / ml into a 50ml beaker and mix to obtain a mixed solution, add 0.3g of ethylene glycol and stir to obtain a gel precursor solution, add Stir 5.4g of activated carbon evenly and place it in the air to dry naturally to obtain a catalyst precursor. Place the catalyst precursor in a muffle furnace and raise the temperature to 300°C at a rate of 20°C / min, keep it for 5 hours and then cool to obtain high catalytic activity. Chlorine-doped iron metal oxide catalysts.
Embodiment 3
[0032] 3.7mg HClO 3 Dissolve 0.464g of cobalt acetate and 0.602g of copper carbonate in 50ml of water, add 0.9g of citric acid, and stir well to obtain a gel precursor solution. Add 3.5g of molecular sieves to the above precursor solution, and then put it in an oven at 100°C drying to obtain a chlorine-doped cobalt-copper-oxygen composite metal oxide precursor. The chlorine-doped cobalt-copper-oxygen composite metal oxide precursor is placed in a muffle furnace and heated at a rate of 40°C / min to 800°C for 2 hours to obtain the chlorine-doped cobalt-copper-oxygen composite metal oxide.
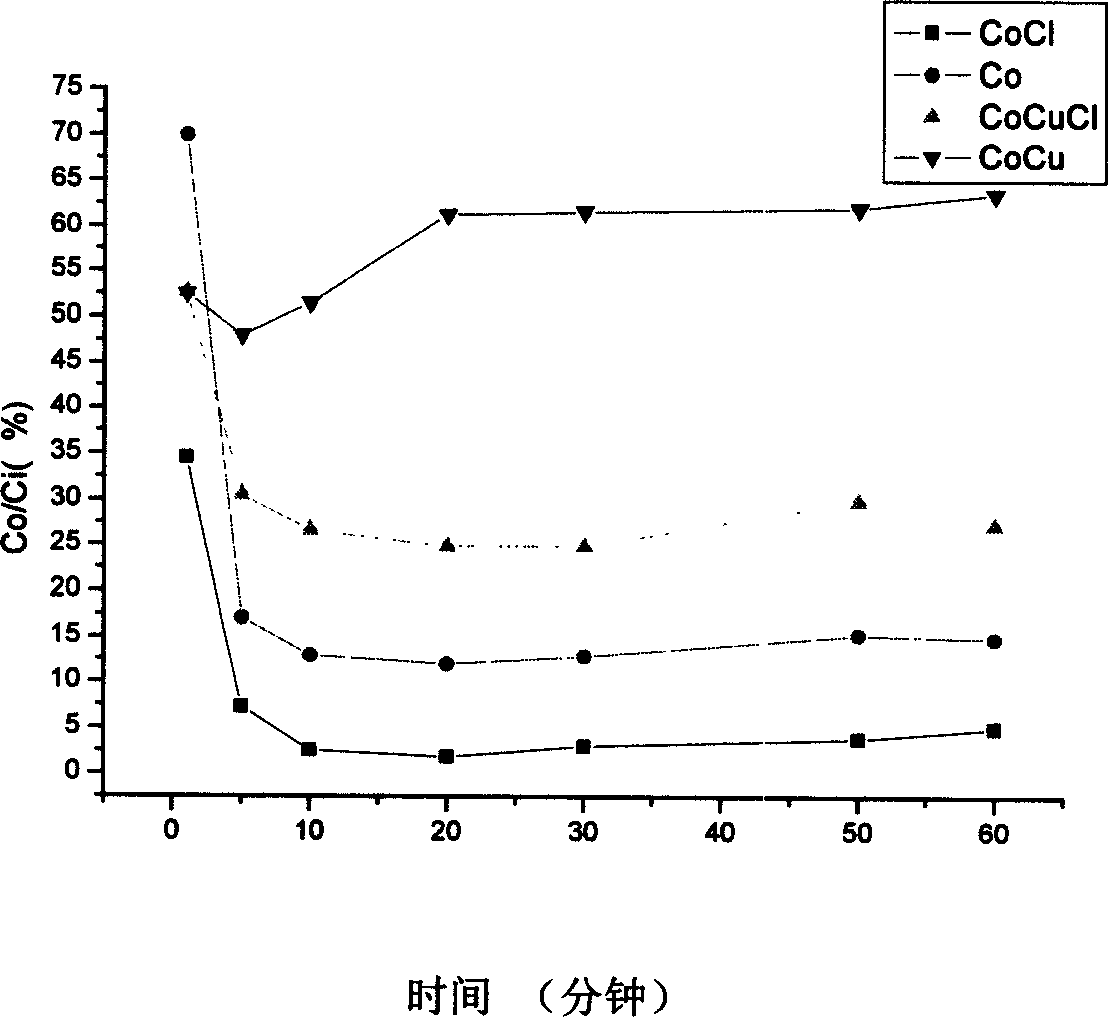
[0033] Catalytic activity test is with embodiment 1, and test result sees figure 1 , where the curve CoCu is the change with time of the ratio of the concentration of element mercury at the outlet of the reaction bed to the concentration of element mercury at the inlet when the catalyst is no cobalt-copper-oxygen composite metal oxide. The ratio of the concentration of elemental mercury at t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com