Gene engineering preparation method of human pancreas secreted trypsin inhibitor
A trypsin inhibition and genetic engineering technology, applied in the field of human pancreatic secretory trypsin inhibitor, can solve the problems of high cost and few sources, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Construction of recombinant expression plasmid pPIC9K-rHuPSTI
[0034] 1. Reagents and materials
[0035] The vector plasmids pPICZαA and pPIC9K were purchased from Invitrogen; the expression strain KM71 was purchased from Invitrogen; the recombinant human pancreatic secretory trypsin inhibitor gene was synthesized by Shanghai Boya Biotechnology Company; restriction enzymes and T4 DNA ligase were purchased from New England company, PCR primers were synthesized by Shanghai Boya Biotechnology Company, and the gel recovery kit was purchased from Omega Biotechnology Company.
[0036] 2. Method
[0037] (1) Synthesis of human pancreatic secretory trypsin inhibitor gene:
[0038] I. Primer synthesis: Due to the long nucleotide sequence of the gene, it is difficult to directly synthesize the target gene. Therefore, after technical and economical considerations, the full-length human gene was amplified by synthesizing four primers and PCR. Pancreatic Secretory Try...
Embodiment 2
[0056] Embodiment 2: Fermentation of Pichia pastoris engineered bacteria KM71 (pPIC9K-HuPSTI)
[0057] 1. Engineering bacteria: engineering bacteria KM71 (pPIC9K-HuPSTI);
[0058] 2. Medium:
[0059] YPD medium for primary seed bacteria activation (g / L): 20g peptone, 10g yeast powder, 20g glucose:
[0060] MGY medium for activation of secondary seed bacteria (g / L): ammonium sulfate 10g, YNB3.4g, glycerol 10g, biotin 4×10 -4 g;
[0061] Fermentation tank fermentation medium:
[0062] Basal medium: (NH4)2SO4 20g / L, glycerol 40g / L, KH2PO4 12g / L, CaSO4·2H2O 1g / L, Histidine 0.4g / L.
[0063] Trace element PTM1: CuSO4.5H2O 6.0g / L, NaI 0.08g / L, MgSO4·H2O 3.0g / L, ZnC12 20.0g / L, HBO4 0.02g / L, FeSO4·7H2O 65g / L, CoCl2 0.5g / L , Biotin 0.2g / L, NaMnO4 2H2O 0.2g / L, H2SO4 4ml / L.
[0064] Induced carbon source: 50% methanol + 25% glycerol (12ml PTM1 per liter)
[0065] 3. Activation of the seed bacterium: get the engineered strains (YPD plate) preserved in -70°C and 20% glycerol, cultiva...
Embodiment 3
[0070] Example 3: Preparation and purification of recombinant human pancreatic secretory trypsin inhibitor:
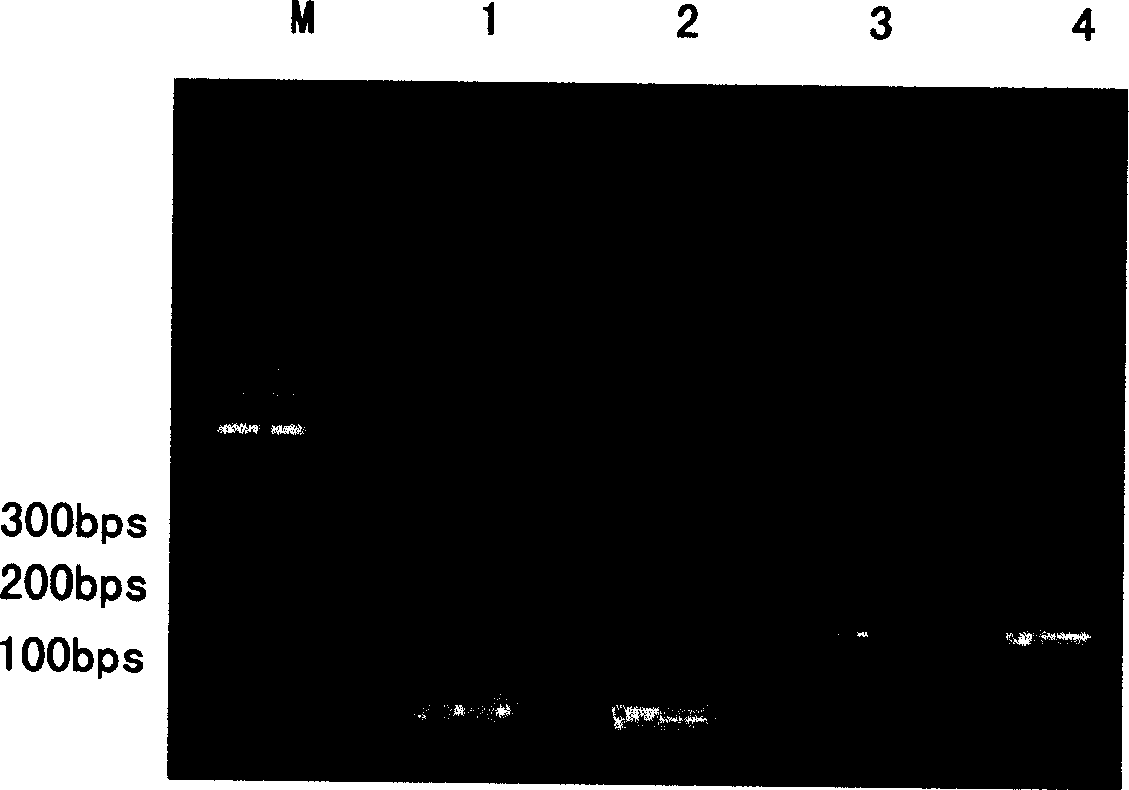
[0071] After using methanol to induce the secretory expression of human pancreatic secretory trypsin inhibitor for 96 hours, the supernatant was collected by centrifugation at 8000 rpm for 15 minutes at 4°C, and the supernatant was collected on Sepherdex G-25 molecular sieve (using 56mM Tris-HCl pH9.0 buffer Equilibrium in advance), collect the protein peak passing through the ultraviolet light, and sample the 15% SDS-PAGE electrophoresis detection result. The desalted and buffer-changed protein solution was loaded onto the Q Sepharose XL chromatography column (pre-equilibrated with 56mM Tris-HCl pH9.0 buffer), and after the UV was eluted to the baseline with the equilibration buffer, the protein peak passing through the UV was collected , sampling 15% SDS-PAGE electrophoresis detection results. Then use buffer 56mM Tris.HCl, 40mM KCl, pH 9.0 for one-step elution, col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com