Process for synthesizing dimethylacetamide by ethyl acetate and dimethylamine
A technology of dimethylacetamide and ethyl acetate is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of high cost, increased investment, and no industrialized reports, etc. The effect of low production cost, reduced corrosion, high industrial application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
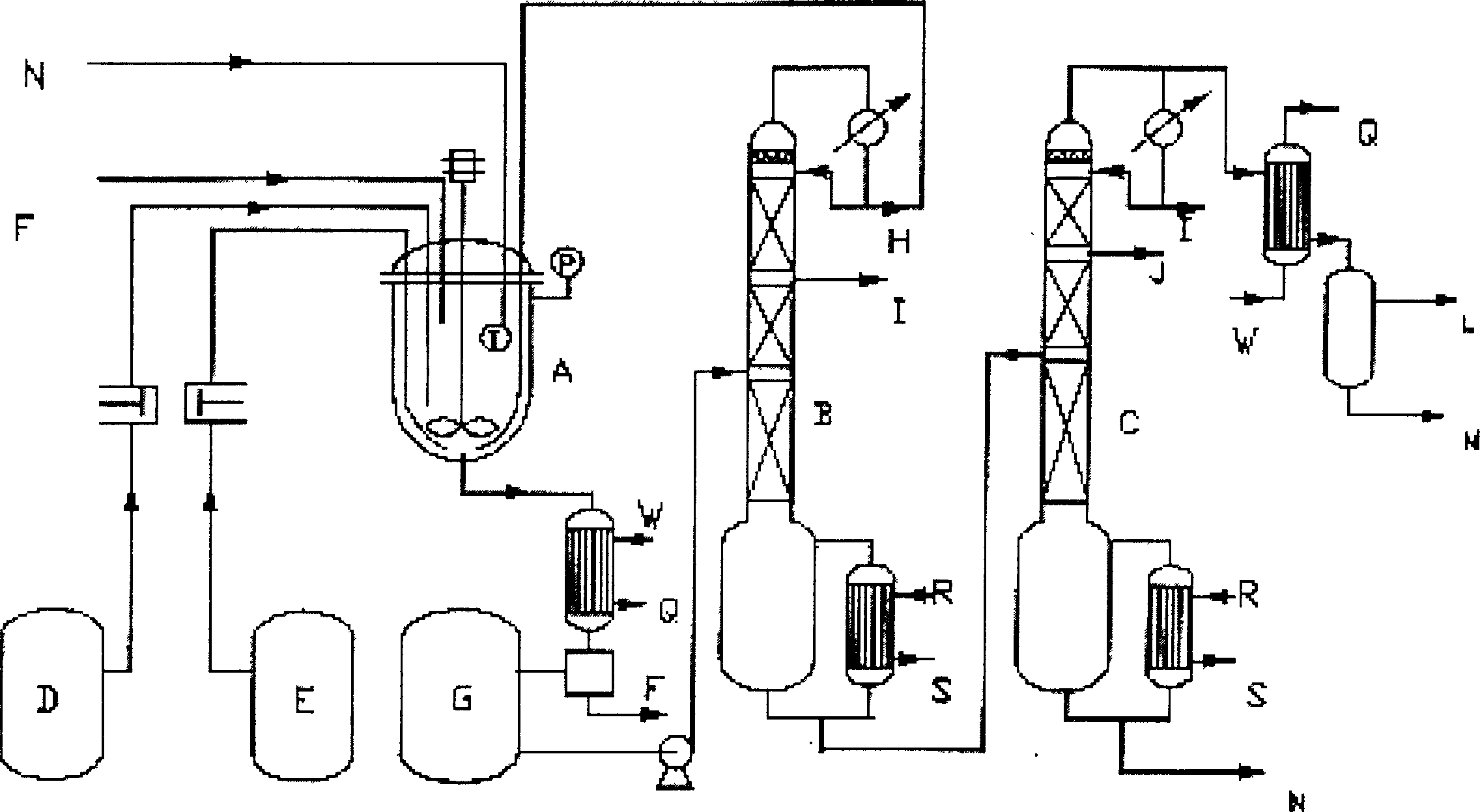
[0020] Add ethyl acetate (720g), dimethylamine (420g), molybdenum trioxide catalyst (3.6g) and tungsten trioxide catalyst (3g) into A (tank reactor), then raise the temperature to 142°C, and naturally raise the pressure to 2.0 Mpa, stirred and reacted for 24 hours, the reaction liquid was cooled and separated to remove the catalyst, and the reaction product was continuously rectified through B (rectification tower I) and C (rectification tower II) to separate dimethylamine, ethyl acetate and ethanol, and the crude distillation product The product N, N-dimethylacetamide can be obtained by rectification. Excess dimethylamine, ethyl acetate and part of ethanol circulate in the system. The conversion rate of ethyl acetate is 92.6%, and the selectivity of N,N-dimethylacetamide is ≥99%.
Embodiment 2
[0022] Add ethyl acetate (720g), dimethylamine (420g) and molybdenum trioxide catalyst (8g) into A (tank reactor), then raise the temperature to 172°C, pressurize to 2.5Mpa, stir and react for 20 hours, and the reaction solution The catalyst is separated and removed by cooling, and the reaction product is continuously rectified through B (rectification tower I) and C (rectification tower II) to separate dimethylamine, ethyl acetate and ethanol, and the crude distillation product is obtained by rectification to obtain the product N, N- Dimethylacetamide. Excess dimethylamine, ethyl acetate and part of ethanol circulate in the system. The conversion rate of ethyl acetate is 92.4%, and the selectivity of N,N-dimethylacetamide is ≥99%.
Embodiment 3
[0024] Ethyl acetate (720g), dimethylamine (420g) and tungsten trioxide catalyst (3g), molybdenum trioxide catalyst (3g) and sodium metavanidate catalyst (3g) were added to A (tank reactor), then the temperature was raised To 165°C, pressurize to 2.2Mpa, stir and react for 20 hours, the reaction liquid is cooled and separated to remove the catalyst, and the reaction product is continuously rectified to separate dimethylamine and acetic acid through B (rectification tower I) and C (rectification tower II) Ethyl ester and ethanol, the crude distillation product is rectified to obtain the product N, N-dimethylacetamide. Excess dimethylamine, ethyl acetate and part of ethanol circulate in the system. The conversion rate of ethyl acetate is 92.8%, and the selectivity of N,N-dimethylacetamide is ≥99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com