Low pyrogen staphylokinase and its preparation method
A technology for staphylokinase and raw materials, applied in the field of preparation of staphylokinase, can solve the problems of unreported purity and endotoxin content, low pyrogen, unreported endotoxin content, etc., and achieves good thrombolytic effect, low pyrogen, and overall quality excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
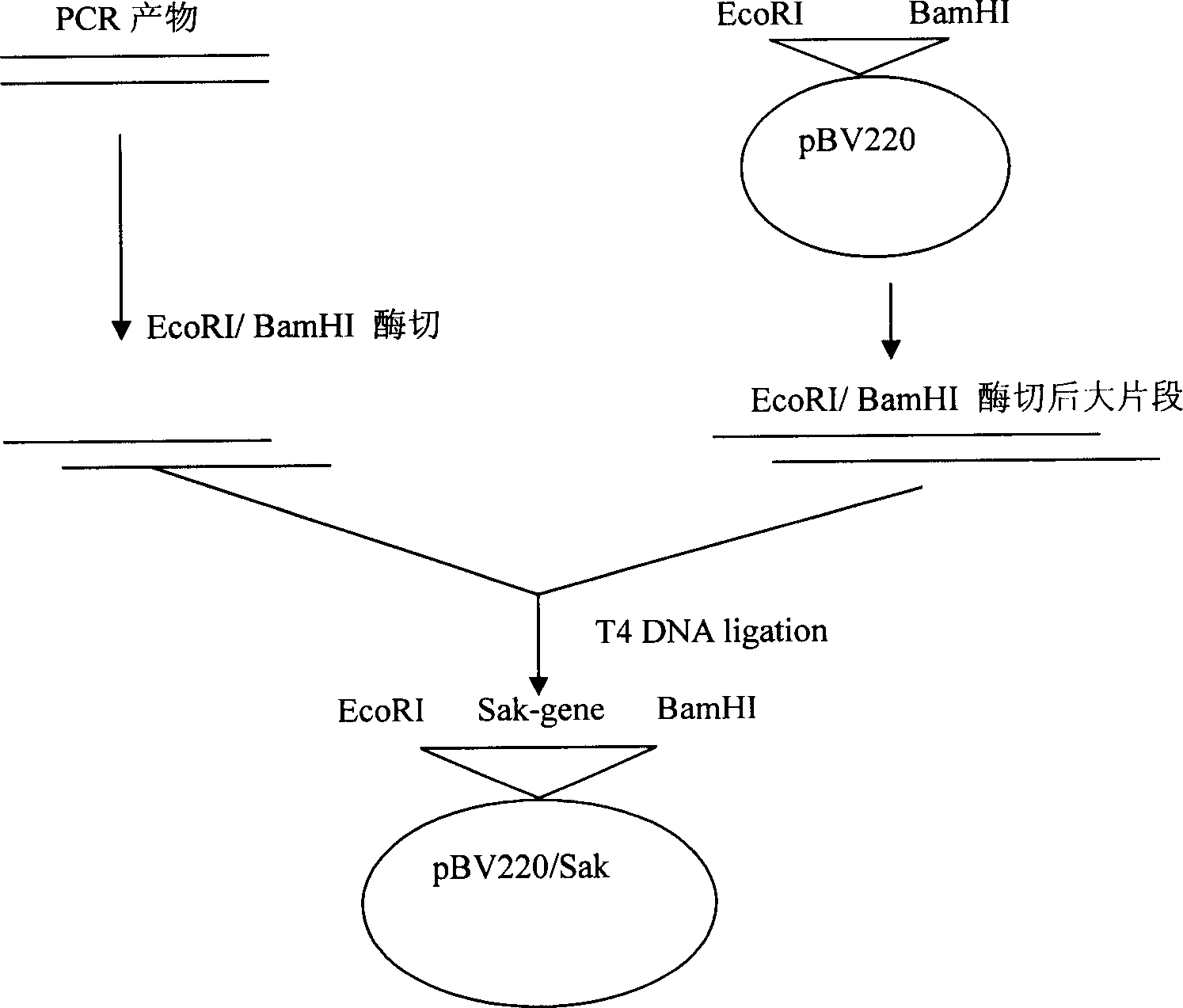
[0053] The preparation of embodiment one engineering bacteria
[0054] Using the complete DNA of Staphylococcus aureus capable of secreting staphylokinase as a template, primers 1 and 2 were used for PCR amplification: Primer 1 (SEQ ID NO.1): 5'GGT GAA TTC ATG TCA AGT TCA TTC GAC AA3';
[0055] Primer 2 (SEQ ID NO. 2): 5'GGA GGA TCC TTA TTT CTT TTC TAT AAC AA 3'.
[0056] Primer 1 comprises EcoR I site, and primer 2 comprises BamH I site, obtains staphylokinase gene, and the DNA sequence (5' to 3 ') of this staphylokinase gene is shown in Table 1:
[0057] Table 1 DNA sequence of staphylokinase gene (SEQ ID NO.3)
[0058] Tcaagttcat tcgacaaagb aaaatataaa 30
[0059] aaaggcgatg acgcgagtta ttttgaacca 60
[0060] acaggcccgt atttgatggt aaatgtgact 90
[0061] ggagttgatg gtaaaggaaa tgaattgcta 120
[0062] tcccctcatt atgtcgagtt tccttattaaa 150
[0063] cctgggacta cacttacaaa agaaaaaatt 180
[0064] gaatactatg tcgaatggga cttagatgcg 210
[0065] acagcatata aagagtttad agtagttgaa ...
Embodiment 2
[0095] The preparation of embodiment two staphylokinase
[0096] 1. Materials and equipment
[0097] Bacteria-breaking tools: homogenizer;
[0098] Ultrafiltration system: Millipore Pellicon 0.5m 2 ×3, the membrane molecular weight cut off is 5000;
[0099] SDS-PAGE electrophoresis scanner: DS-700Bio-Rad;
[0100] Chromatography column: 50×400mm, 100×400mm Shanghai Yarong Biochemical Instrument Factory;
[0101] Chromatography medium: DEAE-Sepharose F.F, CM-Sepharose F.F, and Q-Sepharose F.F are all products of Pharmacia Biotech, and the particle diameters are all 90 μm.
[0102] Chromatographic detection system: UV detector (HD-93-1 type) Shanghai Jinda Biochemical Instrument Factory.
[0103] 2. Preparation process
[0104] The wet weight obtained from fermentation was 1610g engineering bacteria, and 10000ml pH8.010mM phosphate buffer was used to suspend the bacteria, and 9000ml supernatant was obtained after breaking the bacteria. The r-SAK content of the supernatant ...
Embodiment 3
[0114] The preparation of embodiment three staphylokinase
[0115] 1, material and equipment (with embodiment two)
[0116] 2. Preparation process
[0117] Take engineering bacteria with a wet weight of 800g, suspend the bacteria with 5500ml pH8.010mM phosphate buffer, and obtain 5000ml supernatant after breaking the bacteria. The supernatant is scanned by SDS-PAGE electrophoresis. The r-SAK content is 38.9%, and the protein concentration is 10.40 mg / ml, the total protein content is 52000mg, and the r-SAK content is 20228mg.
[0118] The above supernatant was washed with 15% (NH 4 ) 2 SO 4 After precipitation treatment, ultrafiltration (5KD) was used to desalt, and the medium was converted to pH 8.010mM phosphate buffer. Concentrate to 1000ml, protein concentration is 43.4mg / ml (total protein amount 43400mg).
[0119] DEAE-Sepharose F.F permeation chromatography column: 100×400mm (Shanghai Yarong Biochemical Instrument Factory), DEAE-Sepharose F.F column height 300mm, mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com