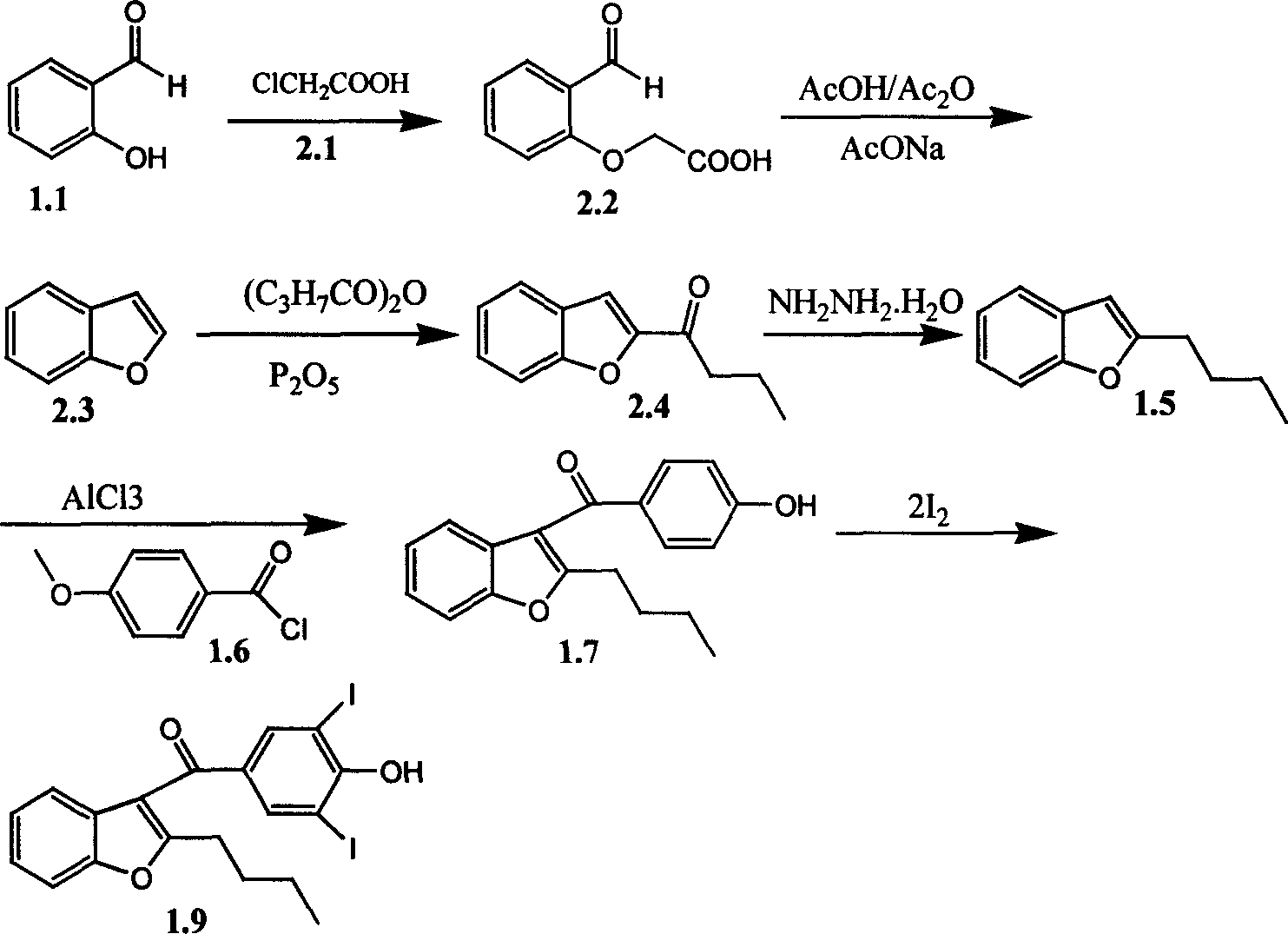
Synthetic process for 2-butyl-3(hydroxy 3,5-diiodo-benzoyl) benzofuran
A technology of butylbenzofuran and diiodobenzoyl, which is applied in the field of synthesis technology of 2-butyl-3-benzofuran, can solve the problems of high pollution, low total reaction yield and high production cost, Achieve the effect of reducing production cost, improving reaction yield and reducing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 400 grams of toluene, 145 grams of potassium carbonate and 209 grams of methyl 2-bromohexanoate into a 2-liter reaction flask, and add 125 grams of salicylaldehyde in batches at room temperature. Heated to reflux and reacted for 2 hours under reflux conditions. After the reaction, the reaction bottle was cooled to room temperature, then 200 grams of water was added to the reaction bottle, and the pH of the liquid in the reaction bottle was adjusted to 1-2 with concentrated hydrochloric acid under stirring. The water layer was separated, and the reactant in the upper layer was washed with water and then refluxed with water in the reaction bottle under normal pressure to obtain the reaction solution.
[0020] The toluene was evaporated from the above reaction liquid, and 300 g of propanol and 200 g of sodium hydroxide were added to the obtained raffinate, and the reaction was carried out under reflux for 10 hours. After the reaction, distill off propanol under reduce...
Embodiment 2
[0023] Add 174 grams (1 mole) of 2-butylbenzofuran and 520 grams of toluene in a 2000 ml reaction flask equipped with a thermometer, a reflux condenser and mechanical stirring. A small amount of water in the reaction system was removed by azeotropic band water. Then the reaction solution was cooled to 20°C, and 174 grams of p-methoxybenzoyl chloride and ytterbium trifluoromethanesulfonate Yb (OYf) 3 50mmol is quickly added to the bottle, and this ytterbium trifluoromethanesulfonate can be fresh or recovered in the experiment. And after maintaining this temperature for 15 minutes, the temperature of the reaction solution was raised to 40°C, and the reaction was stirred at 40-45°C for 2 hours; then the reaction solution was cooled to 0°C, and then 200 ml of ice water was added dropwise to the reaction solution, Control the internal temperature not to exceed 20°C during the dropwise addition; continue stirring at this temperature for 15-30 minutes after the dropwise addition, fi...
Embodiment 3
[0027] 500 grams (1.7mol) of 2-butyl-3-(4-hydroxybenzoyl) benzofuran (99.5%), 124 grams (0.9mol) of potassium carbonate, and 453 grams of iodine (1.78mol) were dropped into 5 liters of reaction In the bottle, add 2 kilograms of 75% propanol, stir to dissolve completely, slowly heat up to 80~85 ℃ and react for one hour, then heat up to reflux, start to drop 214 grams of hydrogen peroxide (technical grade hydrogen peroxide, 27 % content, 1.7mol) and the mixed solution of 170 gram propanols, dripped in 1.5 hours. Continue to keep warm for 2 hours. Cool naturally to room temperature, adjust the pH to 1 with concentrated hydrochloric acid, filter with suction, and wash the filter cake with water to obtain light yellow 2-butyl-3-(4-hydroxy-3,5-diiodobenzoyl)benzofuran, 60 ℃ / 30-50mmHg vacuum drying to obtain 832 grams of dry product, the yield is 89.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com