Sustained-releasing oral mucosa medicinal film
A slow-release patch and oral mucosa technology, which is applied in the direction of drug combination, sheet delivery, and pharmaceutical formulations, can solve the problems of patch falling off, short drug release time, and loss of therapeutic effect of oral diseases Strong, long release time, convenient application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 The oral mucosa drug sustained-release patch adopts the following raw material components and proportions: 8.0 grams of polyvinylpyrrolidone, 8.0 grams of polyoxyethylene, 10.0 grams of microcrystalline cellulose, 1.5 grams of flavoring agent menthol, active pharmaceutical ingredients Minocycline 2.5 grams.
[0025] A flat-bottomed mold with a diameter of 5 mm is used to prepare the tablet directly. The specific preparation method is: uniformly mix the dry powder of the above-mentioned excipients and the dry powder of the active drug according to the formula ratio, and adopt the direct compression conditions of 1000 kg of pressure and 6 seconds. A flat cylindrical patch is made. The thickness of the patch is 0.8-1.2 mm, and the weight of the patch is 25.0-32.0 mg.
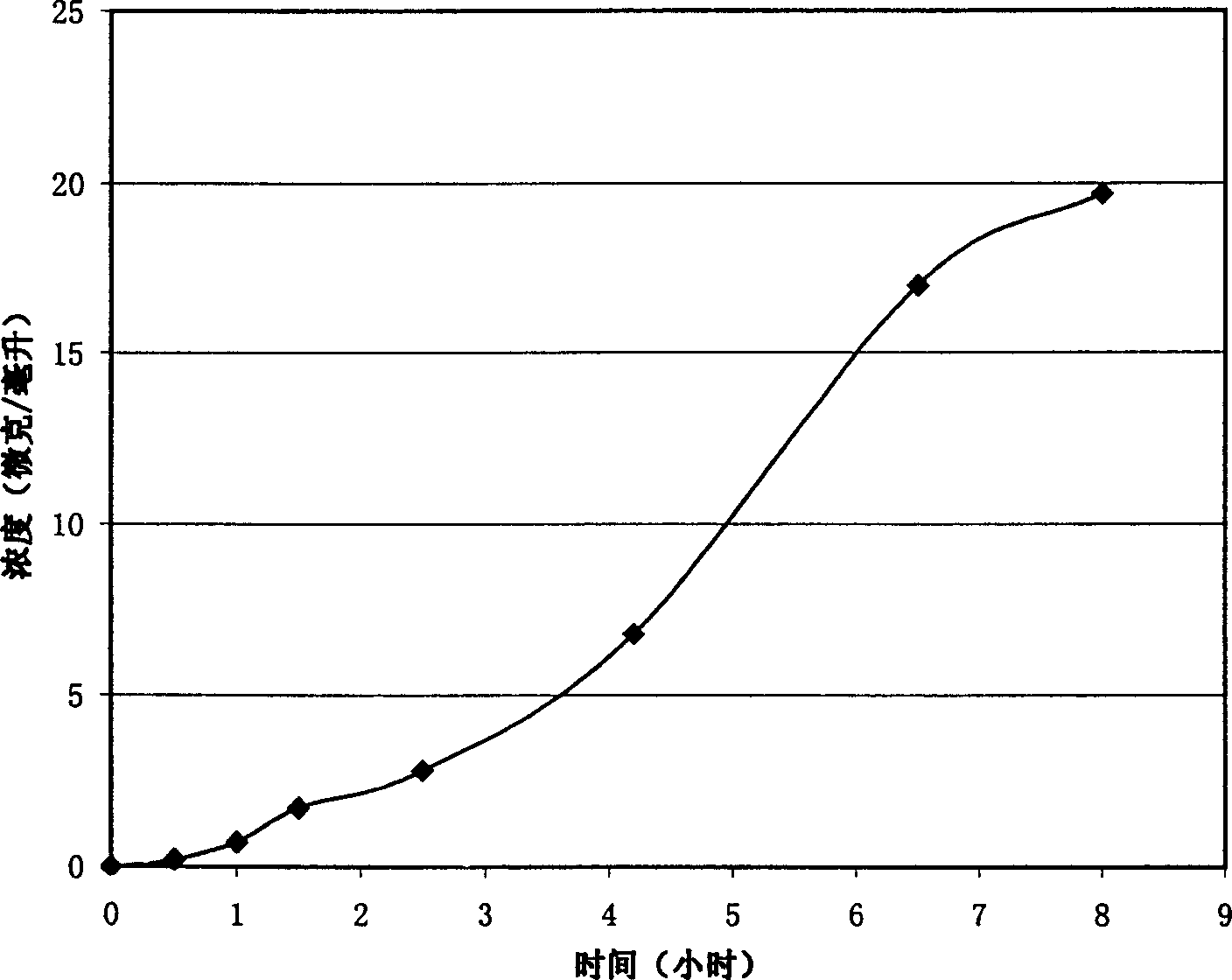
[0026] A piece of the oral mucosa drug sustained-release patch sample was taken for a dissolution test. The diameter of the sample was 5.0 mm, the thickness was 1.2 mm, and the weight was 30 mg. ...
Embodiment 2
[0031] Example 2 The oral mucosa drug sustained-release patch adopts the following raw material components and proportions: 12.0 grams of hydroxyethyl cellulose, 5.5 grams of modified starch, 7.0 grams of microcrystalline cellulose, 0.5 grams of flavoring agent grape essence, effective drug Chlorhexidine 4.0 g.
[0032] A flat-bottomed mold with a diameter of 5 mm was used to prepare the tablet directly. The specific preparation method was the same as in Example 1. A patch sample was taken for an oral disintegration test. The patch had a thickness of 0.8 mm and a weight of 30 mg. The patch is attached to the surface of the gums of the human body, and the patch adheres to the surface of the gums within 3 minutes, completely sticks to the gums after 10 minutes, and completely erodes and disappears after 2 hours.
Embodiment 3
[0033] Example 3 The oral mucosa drug sustained-release patch adopts the following raw material components and proportions: 2.0 grams of methyl vinyl ether-maleic anhydride copolymer, 2.0 grams of polyoxyethylene, 2.0 grams of sodium hydroxymethylcellulose, natural 12.0 grams of starch, 2.0 grams of flavoring agent menthol, and 0.5 grams of effective drug doxycycline.
[0034] A flat-bottomed mold with a diameter of 5 mm was used to prepare the tablet directly. The specific preparation method was the same as in Example 1. A patch sample was taken for an oral disintegration test. The patch had a thickness of 0.8 mm and a weight of 25 mg. The patch is attached to the surface of the gums of the human body. The patch adheres to the surface of the gums within 3 minutes, completely sticks to the gums after 10 minutes, and completely erodes and disappears after 2.5 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com