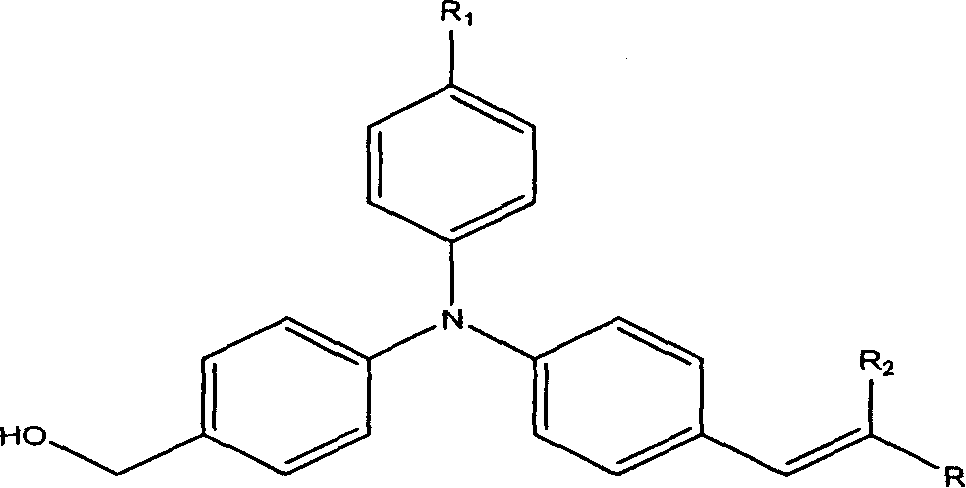
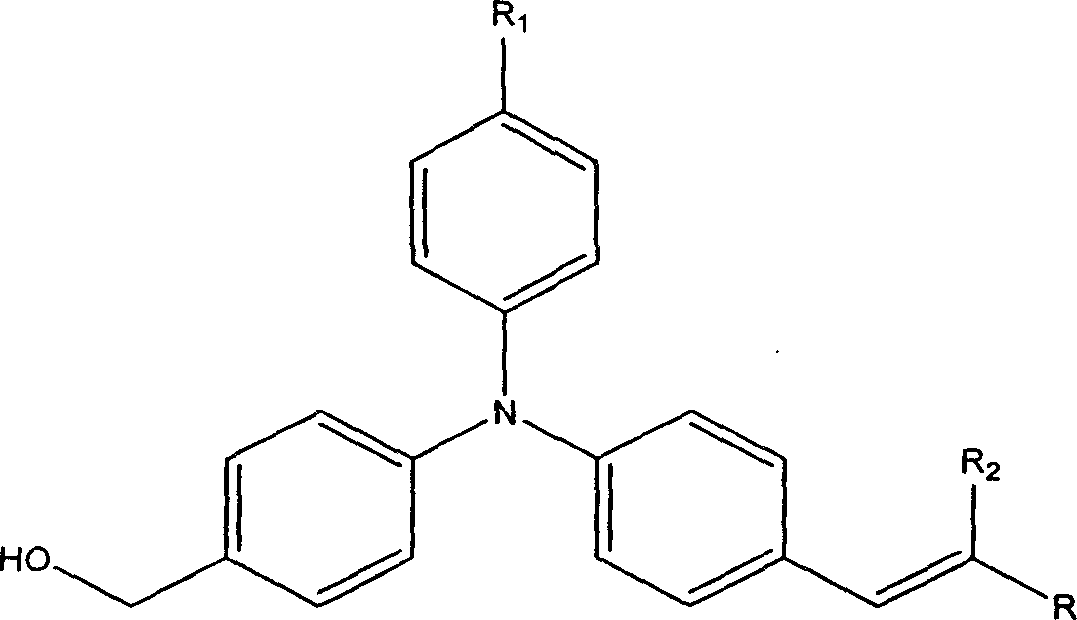
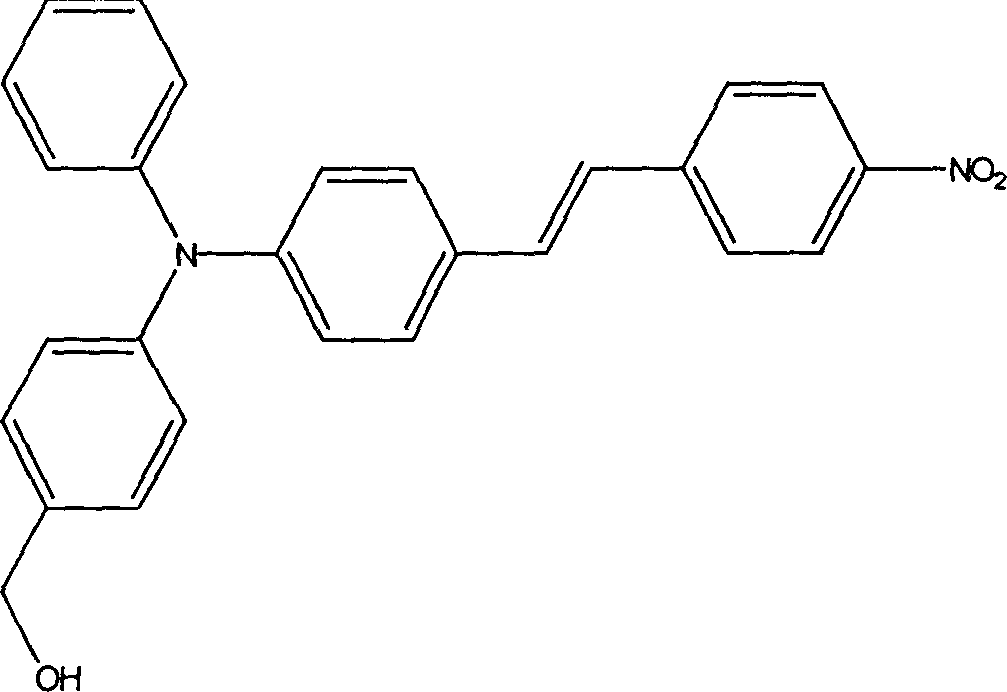
Second-order non-linear optical chromophore containing trianilino group and its synthesis process
A second-order nonlinear, triphenylamine-based technology, applied in the field of D-π-A type second-order nonlinear optical chromophore and its synthesis, can solve the problems of long response time, easy damage, limited application, etc., and achieve high heat The effect of decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of (4-((4-(4-nitrostyryl)phenyl)anilino)phenyl)methanol.
[0058] 1) 17.5ml of phosphorus oxychloride and 13.8g of N,N-dimethylformamide were mixed and stirred in an ice-water bath for 30 minutes, and then 10g of triphenylamine was added. Under the protection of nitrogen, the temperature was raised to 85° C., and the reaction was carried out for 12 hours. After the reaction solution was cooled to room temperature, it was poured into ice-water mixture and stirred vigorously. After the ice cubes melted, 10% NaOH solution was added drop by drop to adjust the pH value to neutral, and placed until a large amount of precipitation occurred. It was extracted with dichloromethane, and the extract was dried with magnesium sulfate and then rotary evaporated to obtain a black-green oily viscous liquid. Peroxide a short column after dissolving in toluene. Concentrate the toluene solution and leave it overnight, filter the solid precipitate and recrystallize t...
Embodiment 2
[0061] Example 2: Synthesis of 3-(4-((4-hydroxymethylphenyl)anilino)phenyl)-2-(4-nitrophenyl)acrylonitrile.
[0062] 1) Prepare 4-((4-hydroxymethylphenyl)phenylamino)benzaldehyde with steps 1) and 2) of Example 1.
[0063] 2) 2.5g of 4-((4-hydroxymethylphenyl)phenylamino)benzaldehyde and 1.7g of p-nitrophenylacetonitrile were mixed and dissolved in 20ml of pyridine, and several drops of piperidine were added for catalysis. The reaction solution was heated to 120°C, reacted for 6 hours under the protection of nitrogen, cooled, poured into the ice-water mixture, extracted with 50ml of dichloromethane after the ice was dissolved, and the extract was repeatedly washed with water and dried. The crude product was subjected to column chromatography with dichloromethane as eluent, and then recrystallized in ethanol to obtain pure chromophore 3-(4-((4-hydroxymethylphenyl)anilino)phenyl)-2- (4-nitrophenyl)acrylonitrile. Yield: 53%. 1 H NMR (500MHz, CDCl 3 , δppm): δ1.69 (s, 2H, -Ph-...
Embodiment 3
[0064] Example 3: 2-(3-(4-((4-hydroxymethylphenyl)anilino)styryl)-5,5-dimethylcyclohexen-2-enylidene)malononitrile Synthesis.
[0065] 1) Prepare 4-((4-hydroxymethylphenyl)phenylamino)benzaldehyde with steps 1) and 2) of Example 1.
[0066] 2) Take 16.5ml of 3,5,5-trimethylcyclohexene-2-enone, dissolve 6.6g of malononitrile in 55ml of N,N-dimethylformamide, add 1.8ml of piperidine , 0.4ml of acetic acid and 0.2g of acetic anhydride as catalysis, stirred at room temperature for 2 hours, heated to 120°C under nitrogen protection, and reacted for 12 hours. After cooling to room temperature, the reaction mixture was poured into 500ml of deionized water, and the crude product was collected by suction filtration and washed with brine, treated with boiling water, and separated by column chromatography (ethyl acetate / petroleum ether=1:12) to obtain light brown solid 2-(3,5,5-trimethylcyclohexene-2-enylidene ) malononitrile. Yield: 56%. 1 H NMR (500MHz, CDCl 3 , δppm): δ1.04(s, 6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com