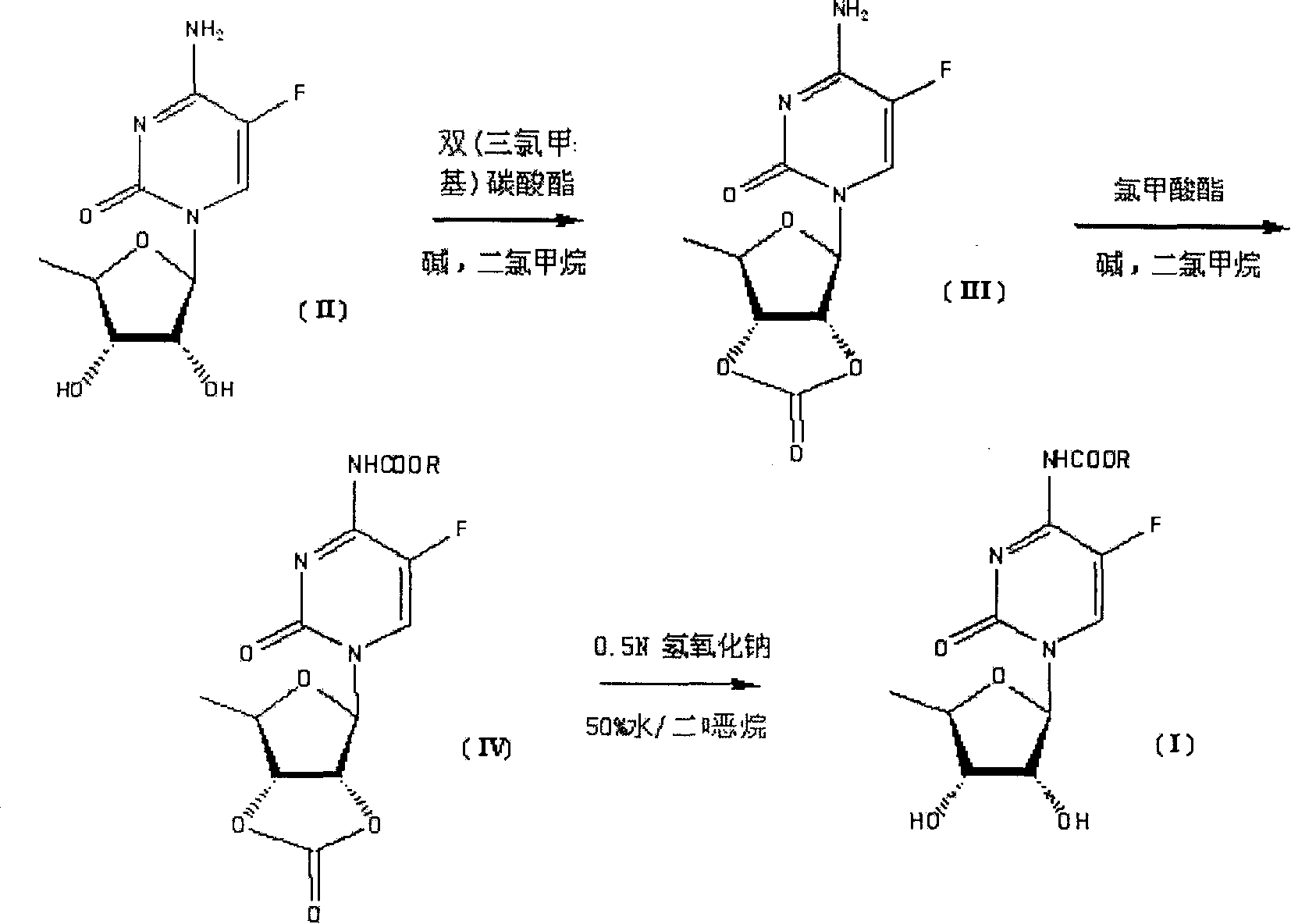
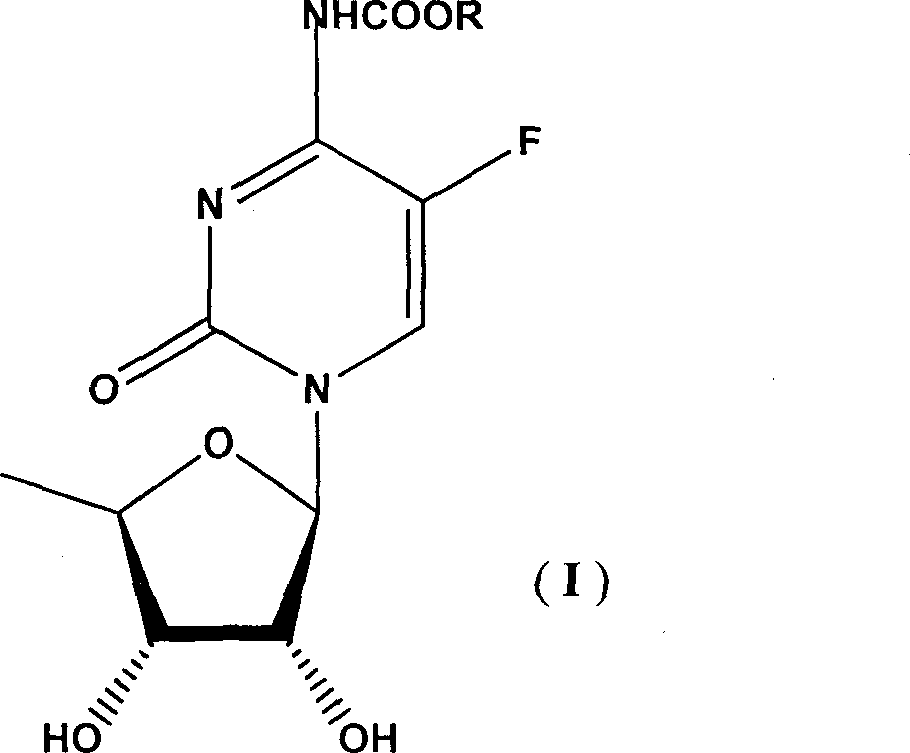
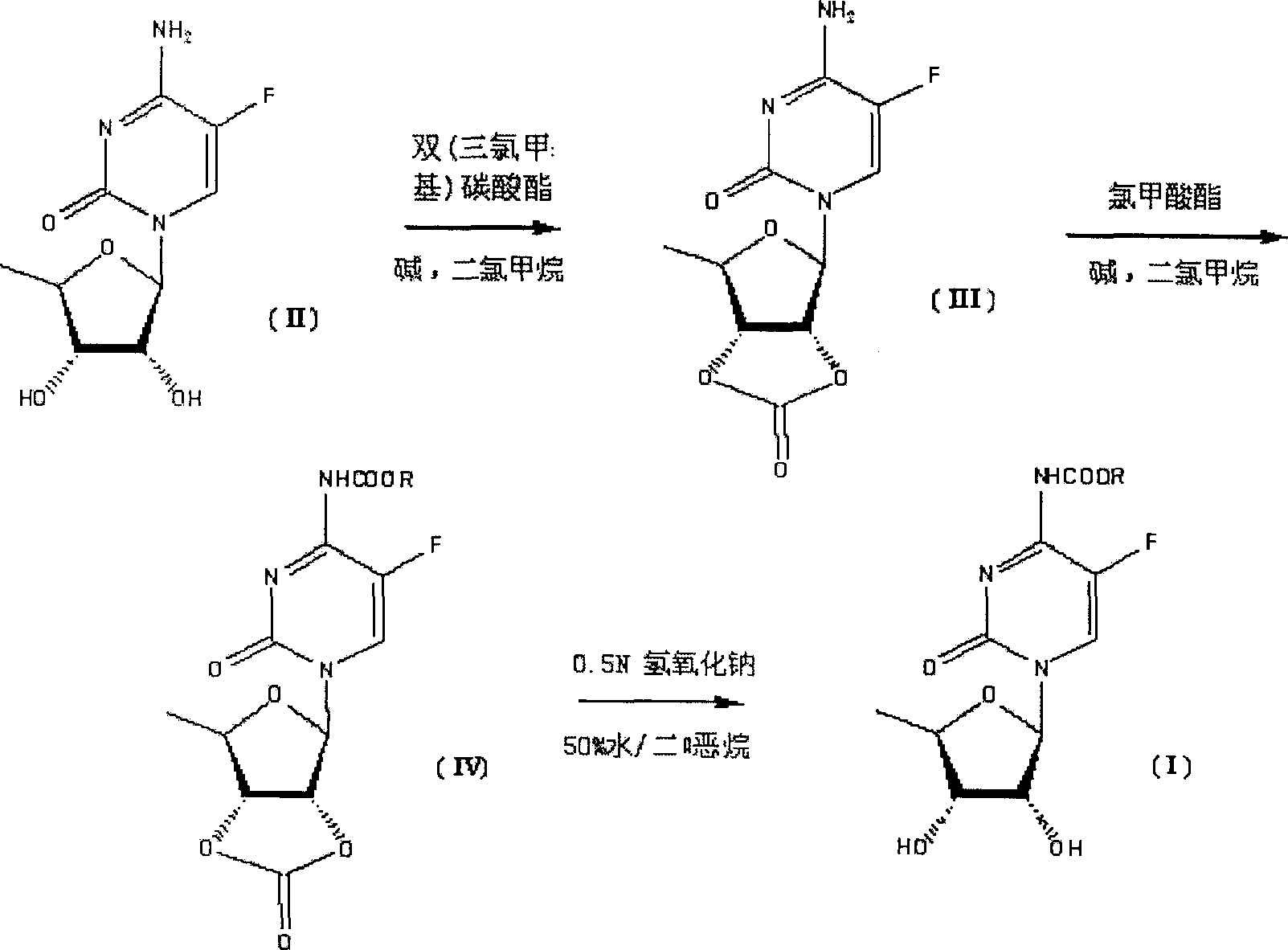
Synthesis of N-acyl-5'-desoxy-5-flucytogly derivative
A technology of flucytidine and its derivatives, which is applied in the field of synthesizing N4-acyl-5'-deoxy-5-fluorocytidine derivatives, which can solve the problems of complex separation and purification operations, consumption of acylating agents, and increased costs. To achieve the effect of abundant sources, short operation time and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0049] The preparation of example 1 compound III
[0050] Dissolve 57.3g of compound II (0.233mol) in 400ml of anhydrous dichloromethane, add 76.0ml of pyridine (0.932mol), add 100ml of a dichloromethane solution of 75.3g of triphosgene (0.256mol) dropwise at room temperature, and drop The mixture was raised to 40°C and stirred for 2 hours. After TLC (EtOAc / petroleum ether=1:7) confirmed that the reaction of the raw materials was complete, the reaction solution was poured into 1000 ml of water, separated, the organic layer was washed with saturated sodium bicarbonate solution, and the aqueous phase was extracted twice with 500 ml of dichloromethane. Combined organic layers, anhydrous Na 2 SO 4 After drying, suction filtration and rotary evaporation, 56.2 g of Compound III was obtained as a light yellow oil, with a yield of 89.0%.
example 2
[0051] The preparation of example 2 compound III
[0052]Dissolve 61.3g of compound II (0.25mol) in 400ml of anhydrous dichloromethane, add 122g of N,N-dimethylaminopyridine (1mol), and add 75.3g of triphosgene (0.256mol) of dichloromethane dropwise at room temperature 100ml of methane solution, the mixture was raised to 30°C and stirred for 2 hours after dropping. After TLC (EtOAc / petroleum ether=1:7) confirmed that the reaction of the raw materials was complete, the reaction solution was poured into 1000 ml of water, separated, the organic layer was washed with saturated sodium bicarbonate solution, and the aqueous phase was extracted twice with 500 ml of dichloromethane. Combined organic layers, anhydrous Na 2 SO 4 After drying, suction filtration and rotary evaporation, 63.7 g of Compound III was obtained as a light yellow oil, with a yield of 94.0%.
example 3
[0053] The preparation of example 3 compound IV
[0054] Dissolve 56.2g of compound III (0.207mol) in 1000ml of anhydrous dichloromethane, add 27.7ml of pyridine (0.340mol), cool to 0°C, and add 93.2g of chloroacetoxy-n-pentane (0.621 mol), the mixture was raised to room temperature and stirred for 2 hours. After TLC (EtOAc / petroleum ether=1:7) confirmed that the reaction of the starting material was complete, the solvent was evaporated under reduced pressure. The residue was poured into 2000 ml ether and saturated aqueous sodium bicarbonate (1 / 1). The organic layer was washed with saturated brine and water, and washed with anhydrous Na 2 SO 4 Dry and rotary evaporate. 59.3 g of compound IV was obtained as a white solid, with a yield of 74.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com