Beta-galactosidase strain, beta-galactosidase and its production process
A technology of galactosidase and production process, which is applied in the field of microorganisms and microbial fermentation, and can solve the problems of fresh milk deterioration, protracted production process, and slow chemical reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the present invention selects the preservation and rejuvenation of bacterial classification
[0039] A strain of lactase-producing bacteria G2005 was isolated from a cold storage in Urumqi, and was identified as Lactococcus. The optimal reaction temperature of the lactase produced was 50°C, and the half-life of the enzyme at 60°C was about 50 minutes.
[0040] Two methods were used for strain preservation, that is, liquid paraffin preservation method and vacuum freeze-drying method. The former is used for short-term preservation of strains (4-6 months), and the latter is used for long-term preservation of strains produced (6 months to several years).
[0041] Activation of preserved strains: take the preserved strains, use an inoculation needle to pick out the strains and inoculate them into a test tube filled with 2 ml of sterile water, and shake for 10 minutes. Then use a sterile pipette to take 0.2 ml and add it to the plate medium TSA (tryptone 15g, s...
Embodiment 2
[0044] Embodiment 2: G2005 lactase activity detection method
[0045] According to FCC standard, fourth edition, July 1, 1996, page 801 to 802 / Lacttase (neutral) / (β-galactosidase) activity (Lacttase (neutral) β-gulactosidase / activity). Lactococcus lactase test method and G2005 lactase characteristics, during the test, the temperature is set at 50°C and the pH is 7.0.
[0046] 1. Definition
[0047] 1 gram of solid enzyme powder (or 1ml of liquid enzyme), under certain temperature and pH conditions, hydrolyzes ONPG (o-nitrophenol galactoside) for 1 minute to produce 1umol tyrosine as an enzyme activity unit, expressed in u / g ( u / ml) said.
[0048] 2. Colorimetry
[0049] 2.1 Principle
[0050] G2005 lactase hydrolyzes ONPG substrate at 50°C and pH 7.0 to produce ONP containing phenolic groups. Under alkaline conditions, ONPG turns yellow, and its enzyme activity is calculated by measuring it with a spectrophotometer.
[0051] 2.2 Reagents and solutions
[0052] 2.2.1 Prep...
Embodiment 3
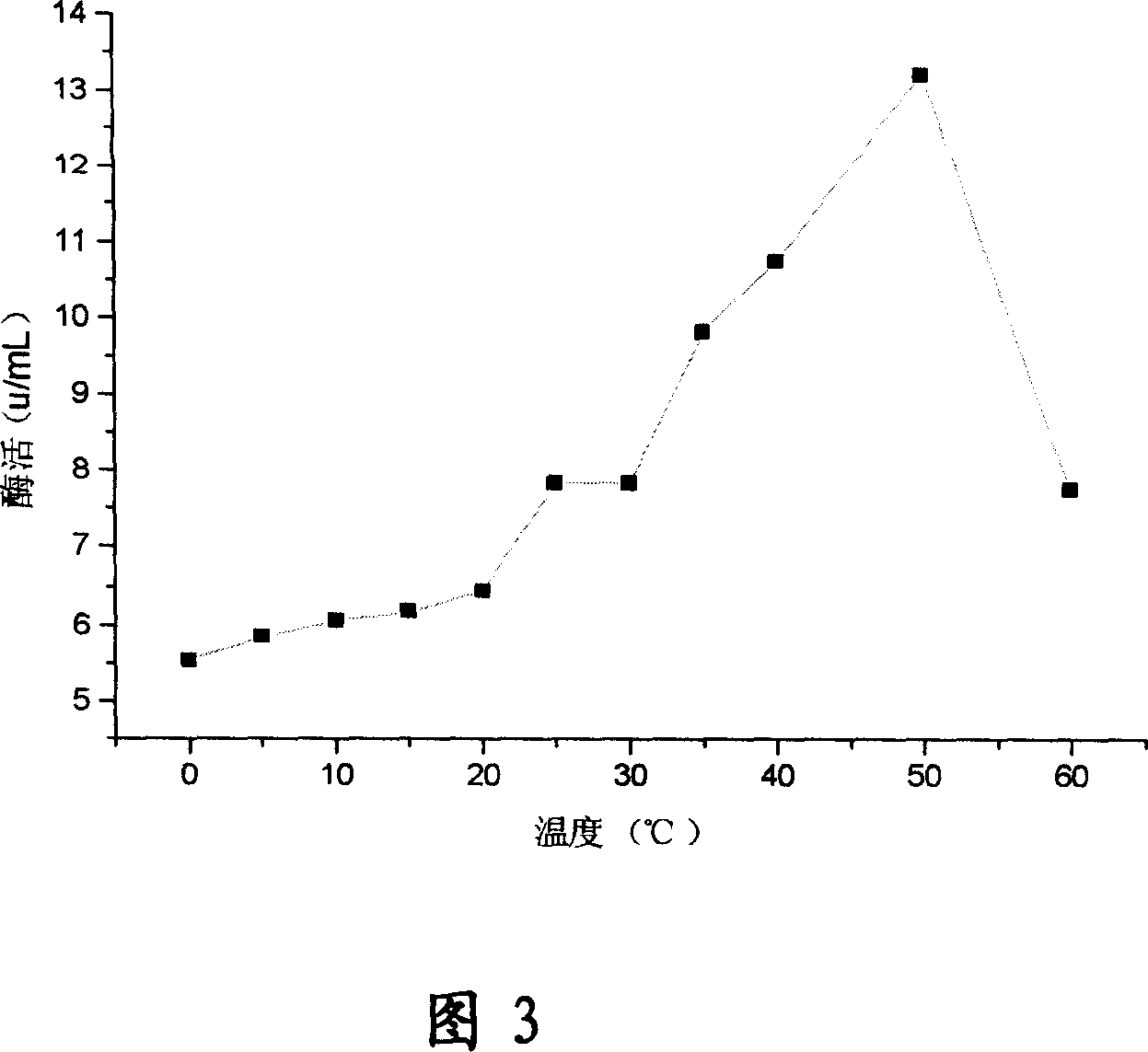
[0086] Embodiment 3: the enzymatic characteristic of strain of the present invention different temperatures
[0087] Medium: tryptone 15g, soy peptone 5g, sodium chloride 5g, lactose 2.5g, dipotassium hydrogen phosphate 2.5g, water 1000ml, pH7.
[0088] At 30°C, 200 rpm, 60ml material / bottle (500ml shake flask), add a cotton plug, incubate for 48h, take 5ml culture suspension in a 50ml centrifuge tube, and use an ultrasonic instrument (broken wall) in an ice-water bath. Break the wall for 5 minutes at an intensity of 10 to release the enzyme from the cells. Take out with a spectrophotometer at a wavelength of 420nm, a 10mm cuvette, and a tube without ONP as a blank, at 0°C, 5°C, 10°C, 15°C, 20°C, 25°C, 30°C, 35°C, 40°C , 50°C, and 60°C were measured for their absorbance, and the calculated enzyme activities were 5.53, 5.86, 6.06, 6.19, 6.46, 7.85, 7.85, 9.83, 10.76, 13.20, and 7.78, respectively. Therefore, the Lactococcus strain (Lacfococcus sp.) of the present invention ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com