Nanocarbon composite structure having ruthenium oxide trapped therein
一种氧化钌、纳米碳的技术,应用在内包氧化钌的纳米碳复合结构体领域,能够解决不能实用、未记载材料容量密度等问题,达到高容量、高电化学活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
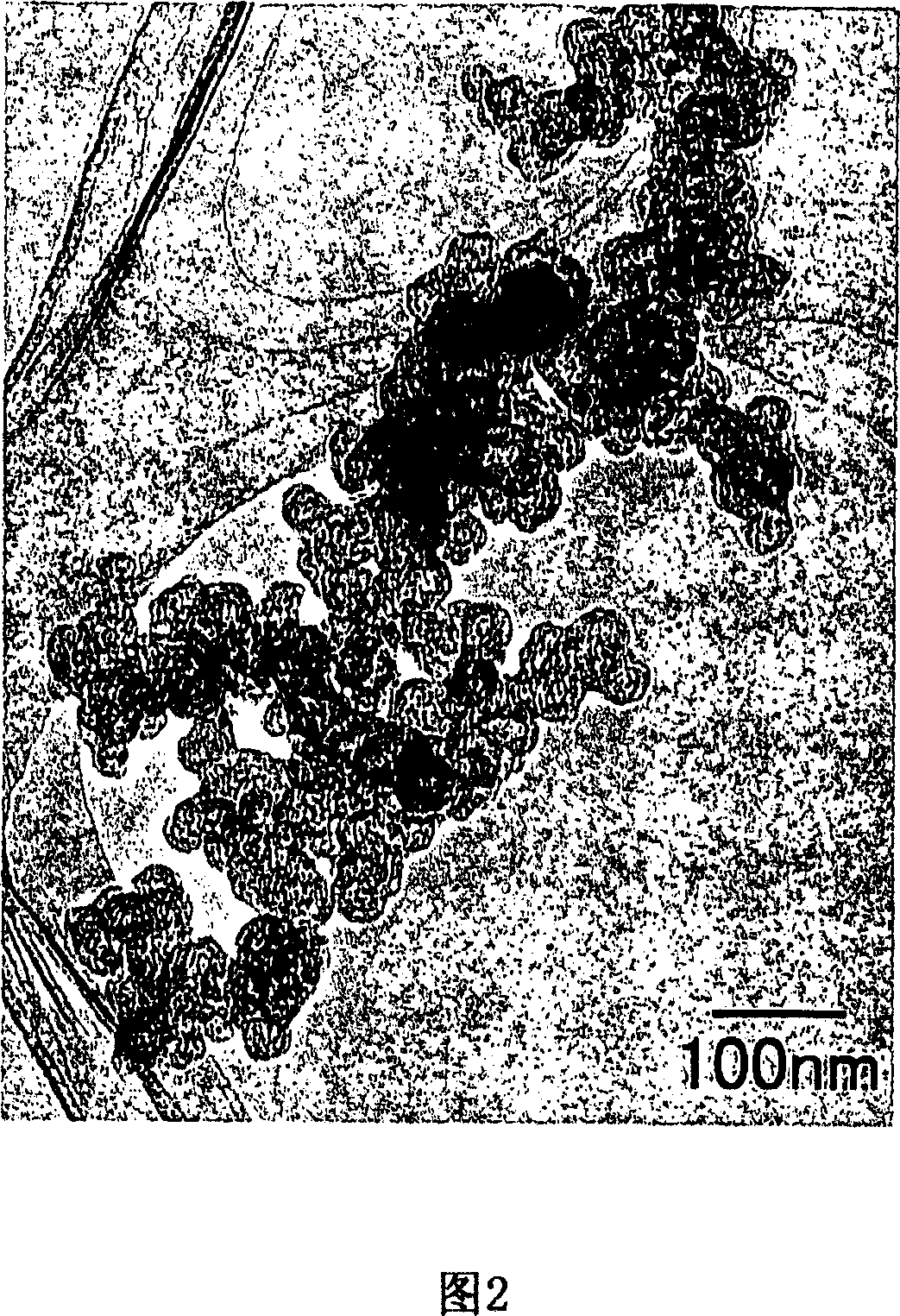
[0063] In a centrifugal treatment device, the above-mentioned hollow nanocarbon (Ketjen Black, manufactured by KETJEN BLACK INTERNATIONAL, trade name: KETJEN BLACK EC600JD, porosity 78 Vol.%, primary particle diameter 40nm, average secondary particle diameter 337.8nm) was highly dispersed , adding a 10 mM ruthenium chloride aqueous solution to it, and continuing to make it highly dispersed, thereby preparing a precursor for adsorbing ruthenium chloride inside and outside the hollow nanocarbon. A 30 mM sodium hydroxide aqueous solution was added thereto to bring the pH to 7, centrifuged at a centrifugal force of 75,000 G for 10 minutes, and a ruthenium oxide-encapsulated nanocarbon composite structure was obtained by performing a surface sol-gel reaction.
[0064] The obtained nano-carbon composite structure containing ruthenium oxide is filtered with an aspirator, a suction filter bottle, and a folded filter, and dried at 100°C for 6 hours to obtain 0.5 hydrated ruthenium oxide...
Embodiment 2
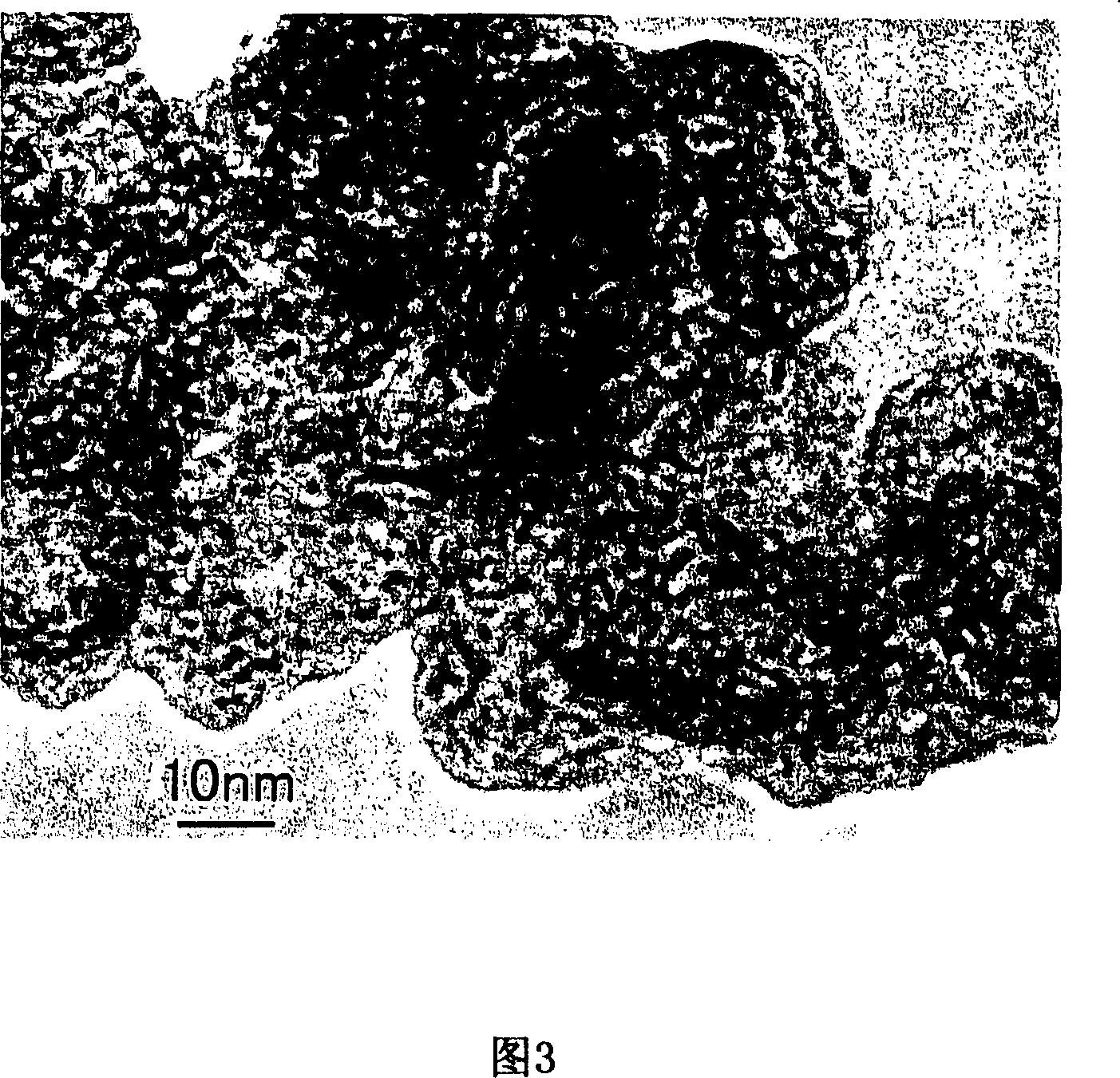
[0067] Except that the centrifugation time of the surface sol-gel reaction in Example 1 was set to 20 minutes, the others were carried out in the same manner as in Example 1 to obtain a nano-carbon composite structure powder (sample B) enclosing ruthenium oxide. The TEM image of sample B is shown in FIG. 4 .
[0068] As can be seen from FIG. 4 , the hollow nanocarbon with a primary particle diameter of about 20 nm forms an aggregate, and its secondary particle diameter is about 200 to 300 nm. Compared with sample A, the ruthenium oxide nanodots (average diameter 1nm) enclosed in the hollow nanocarbon are more cohesive. In addition, the graphene layer forming the primary particle of the hollow nanocarbon is temporarily destroyed and rearranged, so the spherical disintegration.
[0069] [measurement evaluation]
[0070] For the samples obtained in Example 1 (sample A) and Example 2 (sample B), the average particle diameter of the primary particles, the average particle diameter...
Embodiment 3
[0079] Except that the centrifugal treatment in Example 1 was set to 45000G and 10 minutes, it carried out similarly to Example 1, and obtained the nano-carbon composite structure powder (sample C) containing ruthenium oxide. The obtained sample C was evaluated in the same way as sample A and sample B. As a result, the average secondary particle diameter was 380 nm, the average primary particle diameter was 30 nm, and the average particle diameter of ruthenium oxide particles was 10 nm. In addition, the specific electrostatic capacity was 300 F / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent porosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| apparent porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com