Method for determining nerve growth factor content
A nerve growth factor and content technology, applied in the field of nerve growth factor content, can solve the problems such as the limitation of sensitivity of detection methods that cannot exclude human albumin interference, the inability to quantitatively analyze NGF content, and the influence of quantitative determination of NGF, etc. The effect of improving the detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
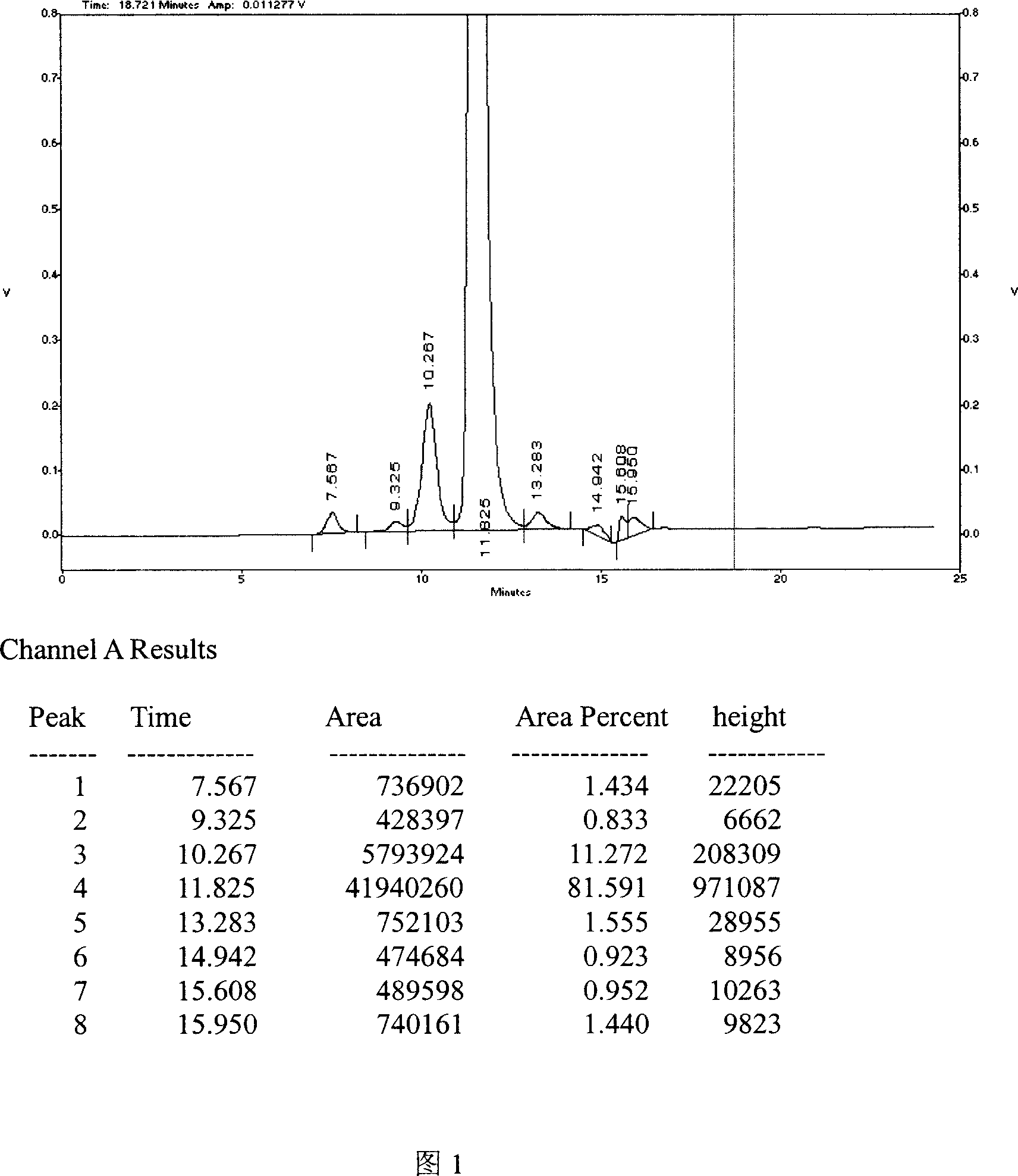
[0035] Example 1: 0.07mol / L sodium dihydrogen phosphate aqueous solution mobile phase was used.
[0036] A chromatographic condition:
[0037] (1) Instrument: use Japan's Shimadzu Shimadzu LC-10Avp high performance liquid chromatograph;
[0038] (2) Gel chromatography column: TSK G3000SWXL 7.8×3.0mm, inner diameter 0.5μm (TOSOH Japan);
[0039] (3) Column temperature: room temperature;
[0040] (4) Detection wavelength: 214nm;
[0041] (5) Flow rate: 0.8ml / min;
[0042] (6) pH value of mobile phase: 7.0.
[0043] Two experimental steps
[0044] (1) Sample processing: Take a nerve growth factor preparation, add 0.5ml of mobile phase precisely, shake gently to dissolve, and use it as the test solution.
[0045] (2) Reference substance treatment: Precisely measure the mouse nerve growth factor reference substance (take the mouse submandibular gland, separate and purify NGF by ion exchange chromatography and molecular sieve, and test the purity according to the method requir...
Embodiment 2
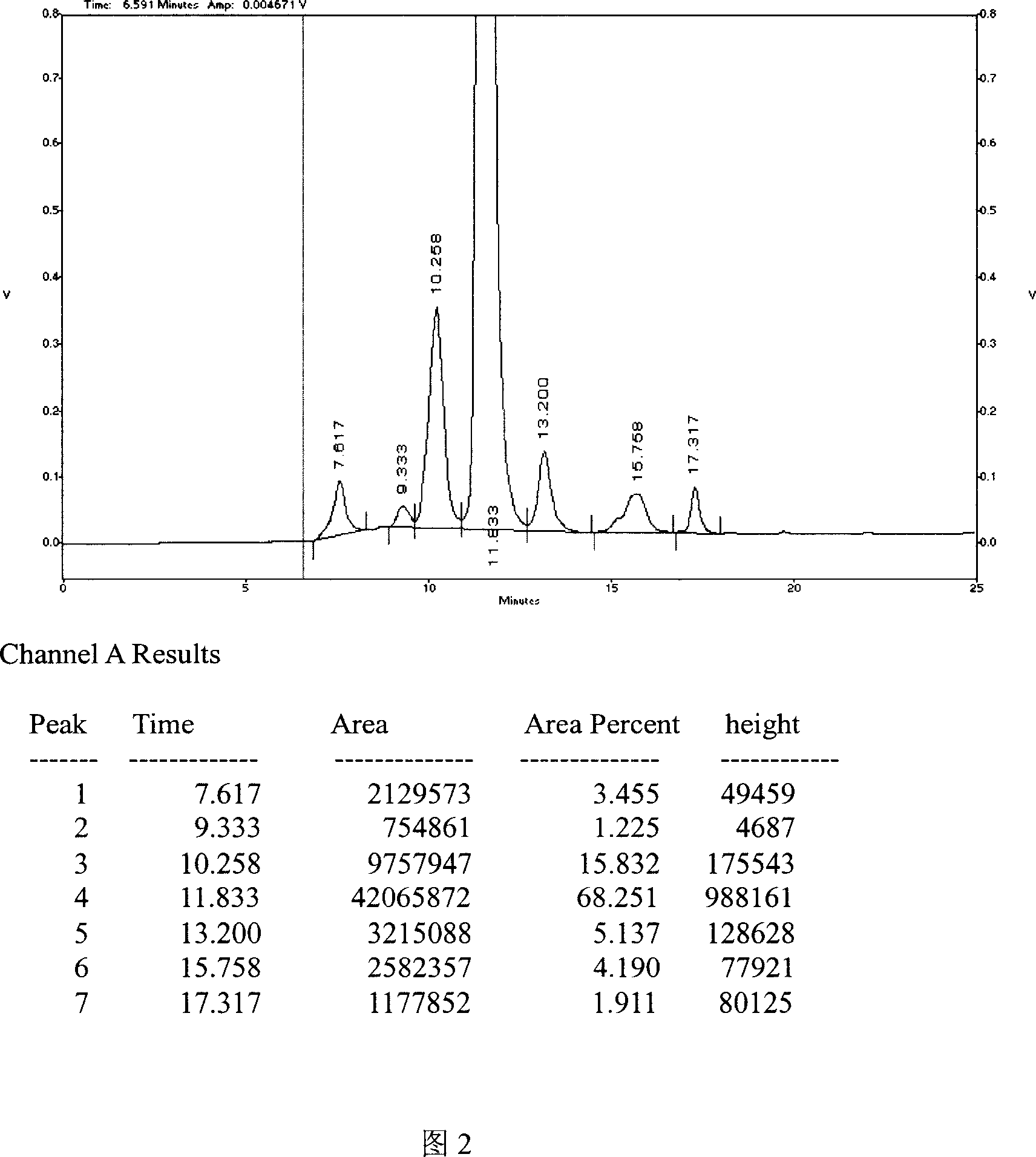
[0048] Example 2: A 2.0 mol / L ammonium acetate aqueous solution was used as the mobile phase.
[0049] 1. Chromatographic conditions: the same as in Example 1.
[0050] Two experimental steps
[0051] (1) The treatment of sample and reference substance is the same as that of Example 1.
[0052] (2) Precisely draw 20 μl of the test solution and the reference solution, respectively, inject them into the liquid chromatograph, and record the chromatogram. Obtain parameters such as retention time, peak height, and peak area.
[0053] Results: The main peak area of nerve growth factor was A=923186, and the area of reference substance was A=921096. The sample content was 100.2% by calculating the peak area by external standard method. The degree of separation between the main peak of nerve growth factor and the main peak of albumin was greater than 1.5, and the retention time of the main peak of the sample was consistent with the retention time of the reference substance. The...
Embodiment 3
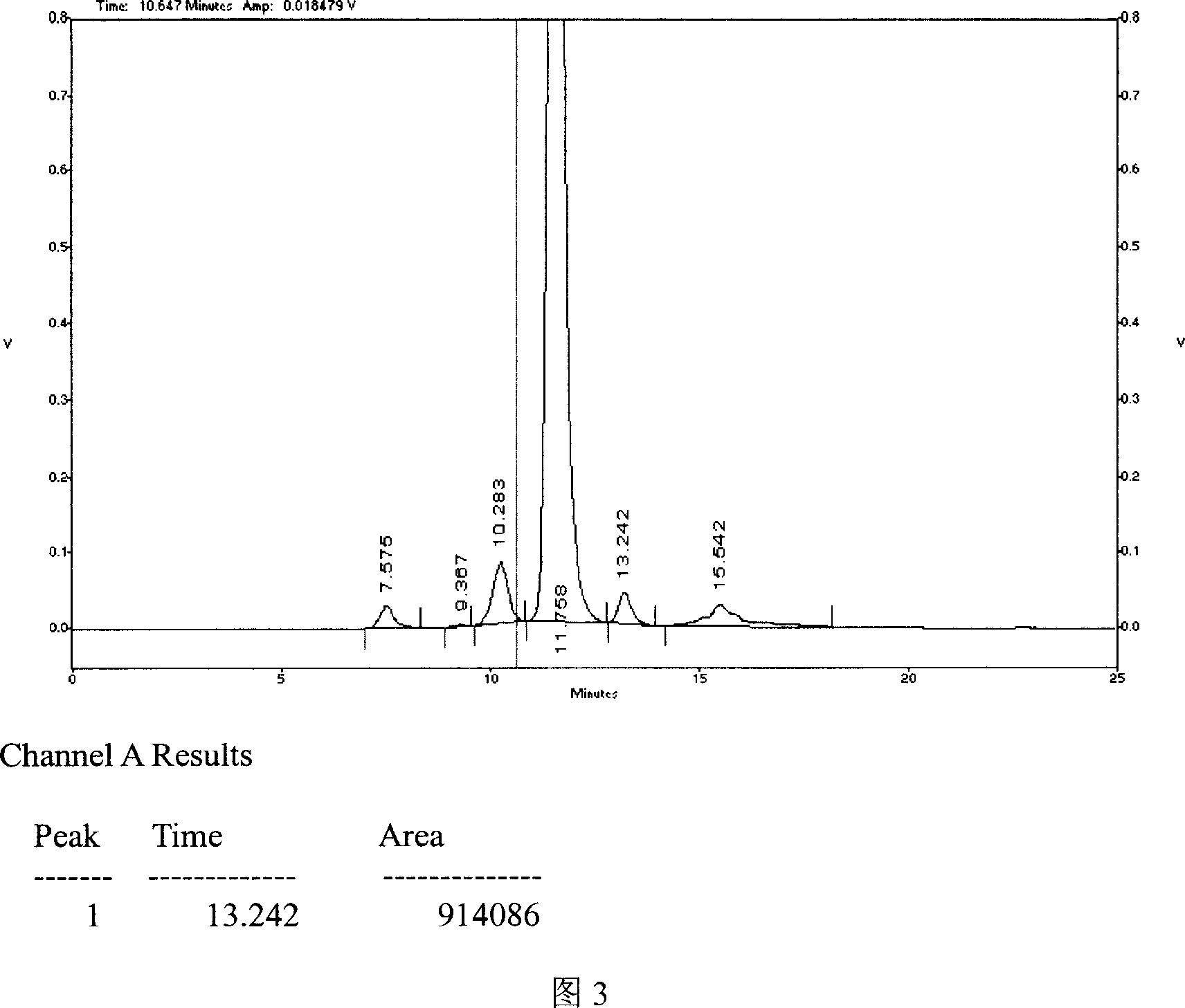
[0054] Example 3: A 0.10 mol / L sodium sulfate aqueous solution was used as the mobile phase.
[0055] 1. Chromatographic conditions: the same as in Example 1.
[0056] Two experimental steps
[0057] (1) The treatment of sample and reference substance is the same as that of Example 1.
[0058] (2) Precisely draw 20 μl of the test solution and the reference solution, respectively, inject them into the liquid chromatograph, and record the chromatogram. Obtain parameters such as retention time, peak height, and peak area.
[0059] Results: The main peak area of nerve growth factor was A=3147076, and the area of reference substance was A=3098921. The sample content was 101.6% calculated by the peak area by external standard method. The degree of separation between the main peak of nerve growth factor and the main peak of albumin was greater than 1.5, and the retention time of the main peak of the sample was consistent with the retention time of the reference substance. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com