The euphorbia lunulate bge extractant and production method and use thereof
A technology of cat's eye grass and extract, applied in the field of cat's eye grass extract and its preparation and application, to achieve the effect of overcoming toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 .The preparation method of cat's eye grass extract
[0029] (1) The crude extraction method and product of cat's eye grass:
[0030] Take 9.0 kg of cat's eye grass (Euphorbia lunulata Bge), extract three times with 70% acetone (27 L), concentrate the extract, add water to the obtained extract to make it a suspension, and extract with chloroform, ethyl acetate, and n-butanol in sequence. The NO formation inhibition test and MTT test were carried out on these three extracts respectively, and the sample concentration was 100 μg / ml. The results are shown in Table 1:
[0031] Table 1. Yield, NO production inhibition rate and MTT toxicity of each extract
[0032] Extracts
Yield (g)
NO production inhibition rate
MTT toxicity
Chloroform extract
65.25
89.8%
have
Ethyl acetate extract
52.43
82.1%
none
n-butanol extract
86.51
33.1%
none
[0033] Select the ethyl acetate extrac...
Embodiment 2
[0044] Example 2 .Physical and chemical properties of main compounds
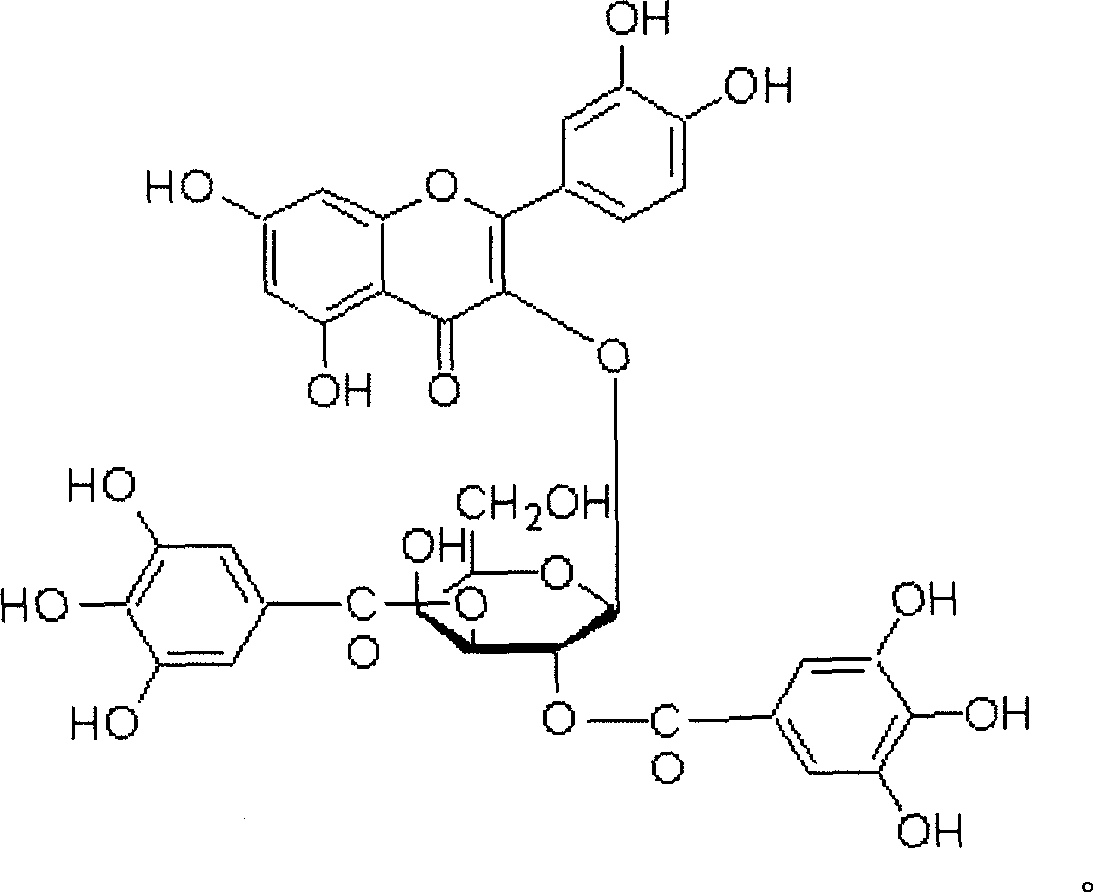
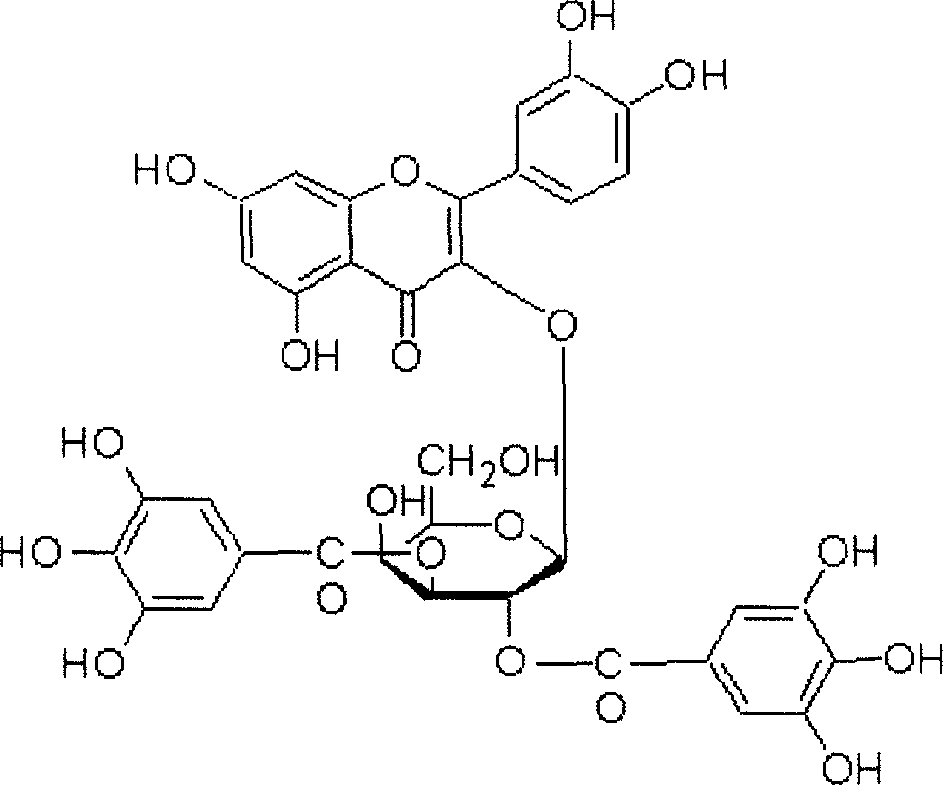
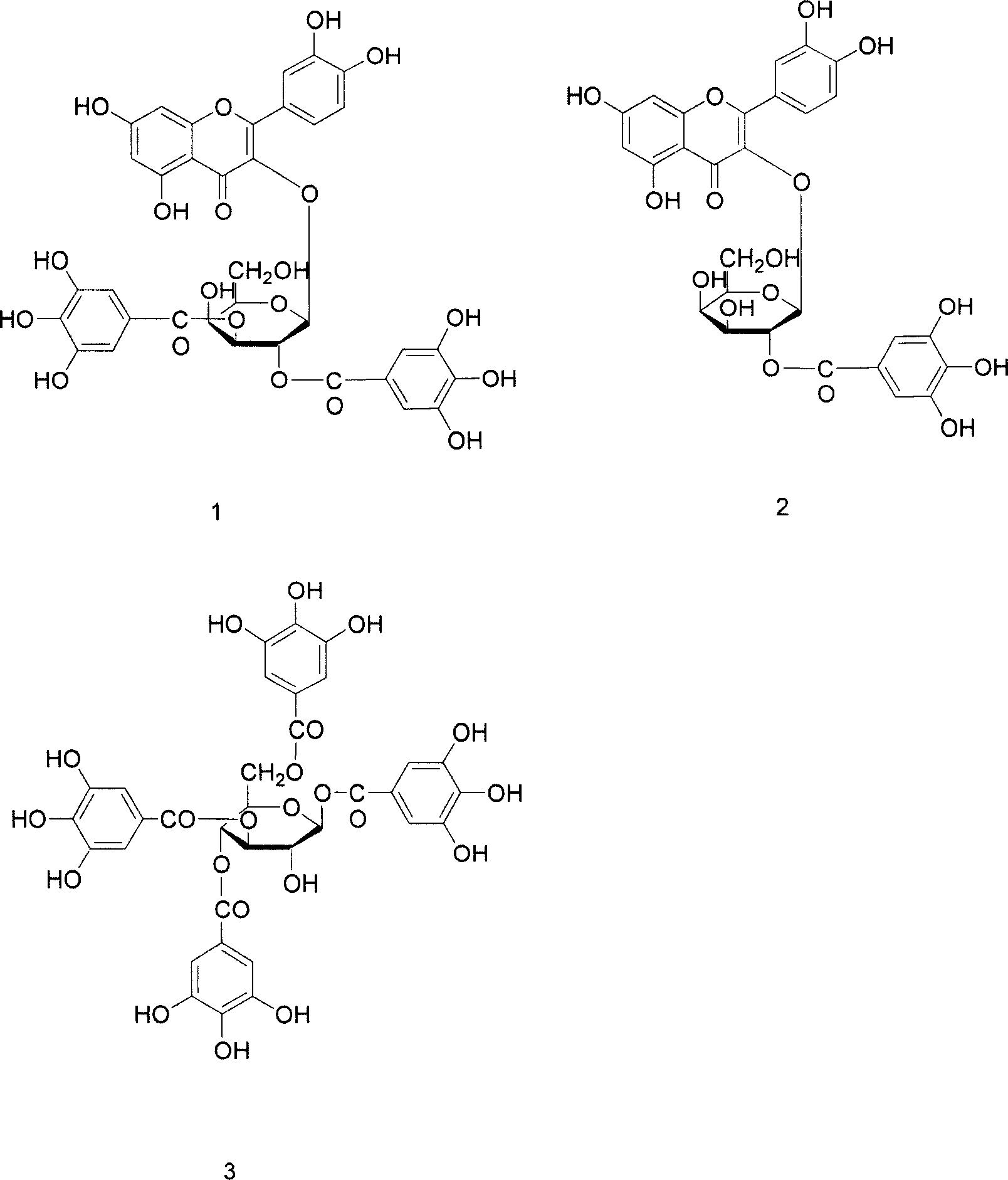
[0045] The physical and chemical parameters of the three compounds obtained by separation and purification were analyzed, and the main data are shown in Tables 3, 4 and 5.
[0046] Table 3. Basic physical and chemical properties of compounds 1-3:
[0047] Compound 1
Compound 2
Compound 3
character
yellow needle powder
yellow needle crystal
HR-MS(m / z)
Negative FAB
767.1087
767.1095
PositiveFAB
617.1096
617.1089
Negative FAB
787
molecular weight
768
616
788
molecular formula
C 35 h 27 o 20
C 28 h 24 o 16
C 38 h 43 o 14
UV lambda nm max (lgε)
269(4.17)
356(3.87)
267(4.20)
350(3.82)
230(4.21)
IR v cm-1 max
3391(OH)
1714 (C=O)
1654
1507(C=C)
3401(OH)
1712 (C=O)
1650(C=C)
3509(OH) ...
Embodiment 3
[0054] Example 3 .NO production inhibitory activity of cat's eye extract
[0055] Materials used:
[0056] RAW264.7 cells (macrophages, transformed with Abelson leukemia virus) were obtained from ATCC (American Type Culture Collection), USA. Ham's F12 medium containing 10% FBS in 5% CO 2 , cultured at 37°C.
[0057] IFN-γ: Genzyme / Techne Company; LPS: Sigma Company; Sulphonamide: Wako Company; N-1-Naphthethylene-diamine Dihyrochloride: Wako Company Griess reagent: (1) N- 1-naphthaleneethylenediamine hydrochloride was dissolved in 5ml of water for injection;
[0058] (2) Add 250 μl of phosphoric acid to 5 ml of water for injection and 50 mg of sulfonamide.
[0059] Instrument: Microtiter plate reader (Microtiter Plate Reader Model 3550, BIO-RAD).
[0060] Specific methods and results:
[0061] Take 50ml of RAW264.7 cells and put them in a Falcon tube for centrifugation (1000rpm, 3min, 4°C) to pellet the cells, remove the supernatant with a pipette, add 10ml of new mediu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com