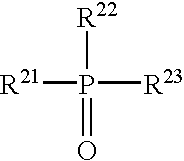
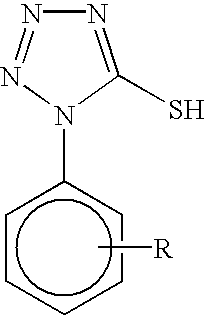
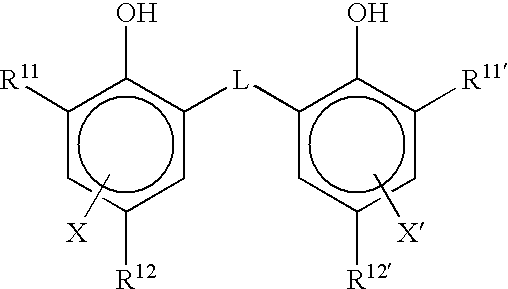
Heat developable image recording material
a technology of developing photosensitive materials and recording materials, which is applied in the direction of photosensitive materials, electrographic processes, instruments, etc., can solve the problems of deterioration of the preservation property of row photosensitive materials, no satisfactory output system for obtaining medical images, and sensitivity decrease during preservation, etc., to achieve excellent image preservation properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0340] (Preparation of PET Substrate)
[0341] PET with a specific viscosity IV of 0.66 (as measured in a mixed solvent of phenol and tetrachlotoethane in a ratio of 6:4 (mass ratio) at 25.degree. C.) was obtained by a conventional method using terephthalic acid and ethylene glycol. The polymer was formed into a pellet, dried at 130.degree. C. for 4 hours, and was extruded from a T type die after melting at 300.degree. C. followed by quenching, thereby obtaining a non-drawn film with a thickness of 175 .mu.m after heat curing.
[0342] The film was drawn 3.3 times using three rolls with different circumference speeds with each other in the longitudinal direction, and subsequently drawn 4.5 times in the transverse direction using a tenterhook. The temperatures for both draw treatments were 110.degree. C. and 130.degree. C., respectively. After heat-curing the film at 240.degree. C. for 20 seconds, it was relaxed by 4% in the transverse direction at the same temperature. After slitting the ...
example 2
[0467] >
[0468] The heat developable image recording material-19 was prepared by the same method as in the heat developable image recording material-9, except that the emulsion layer (photosensitive layer) application fluid-9 was changed to the emulsion layer application fluid-19, and the yellow pigment compound 15 was eliminated from the halation preventive layer.
[0469] The amount of application (g / m.sup.2) of each compound in the emulsion layer was as follows.
5 organic acid silver salt dispersion F 6.19 pigment (C. I. Pigment Blue 60) 0.036 organic polyhalogen compound-2 0.13 organic polyhalogen compound-3 0.41 phthalazine compound-1 0.21 SBR latex 11.1 reducing agent complex-3 1.54 mercapto compound (1-17) 0.002 silver halide (as Ag) 0.10
[0470] The heat developable image recording materials-11 to 18 and 20 were prepared by the same method as in the heat developable image recording material-19, except that the organic acid silver salt dispersion F of the heat developable image reco...
example 3
[0473] >
[0474] The emulsion layer (photosensitive layer) application fluid-9 in the heat developable image recording material-9 was changed to the emulsion layer (photosensitive layer) application fluid-29, and the yellow pigment compound 15 was eliminated from the halation preventive layer. The fluorine based surfactants F-1, F-2, F-3 and F-4 in the protective second layer and back face protective layer were changed to F-5, F-6, F-7 and F-8, respectively, with the same weight. The heat developable image recording material-29 was prepared by the same method as in the heat developable image recording material-9 except the conditions above.
[0475] The amount of application (g / m.sup.2) of each compound in the emulsion layer was shown in Table 4.
7 organic silver halide dispersion F 5.57 pigment (C. I. Pigment Blue 60) 0.032 reducing agent-4 0.40 reducing agent-5 0.36 organic polyhalogen compound-2 0.12 organic polyhalogen compound-3 0.37 phthalazine compound-1 0.19 SBR latex 10.0 hydroge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon number | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com