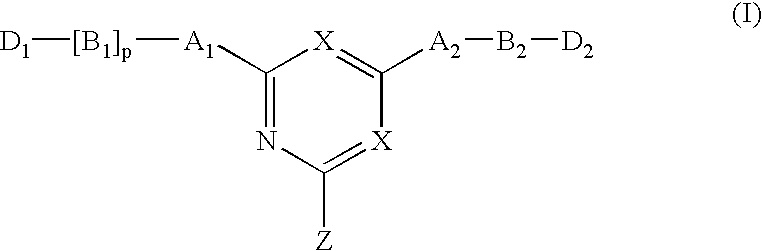
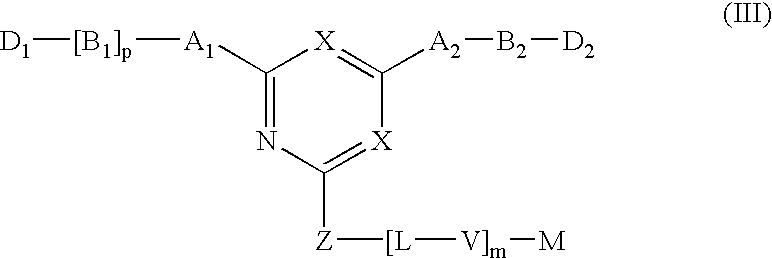
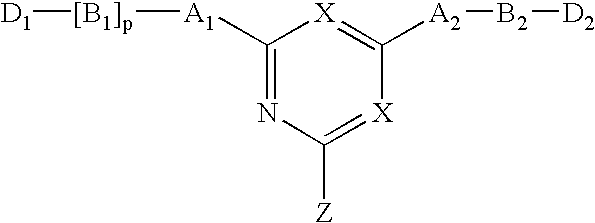Novel triazine-based detoxification agents and their use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
This Example illustrates the synthesis of a typical affinity ligand of General Formula (XIV) defined by the reaction of a halogenohetercyclic compound of General Formula (IV) with a compound of General Formula (V) and (VI).
A solution of 1 part cyanuric chloride in 10 parts acetone was added dropwise to a stirred solution comprising 2 parts L-arginine in 100 parts water. The mixture was stirred for 2 hours at 0-5° C. whereupon the solution was warmed to 30° C. and mixing continued for a further 16 hours. The pH was maintained within the range 5.0-7.0 throughout by titration with 1M sodium hydroxide solution. The reaction product was precipitated by the addition of solid sodium chloride to a final concentration of 20% (w / v), filtered and dried in-vacuo. TLC analysis (THF / propan-2-ol / water (1:2:1 by vol.) solvent) revealed the presence of a single reaction product (Rf 0.42). The molecular mass of the isolated compound was determined by mass spectroscopy and found to be consistent wi...
example 2
This Example illustrates the synthesis of an optionally derivatised support matrix of General Formula (VI).
A solution of 1 part 1,6-diaminohexane in 12 parts water was added to a stirred suspension comprising 29 parts epoxy-activated agarose beads (30 μmol epoxide groups per g agarose gel) in 48 parts water and stirred for 24 h at 30° C. The amino-hexyl agarose gel was filtered and washed consecutively with 12×29 parts water and allowed to drain under gravity on completion of the final wash. Analysis of the resulting amino-hexyl agarose for the presence of primary amines (TNBS asay) and epoxide groups (thiosulphate / sodium hydroxide titration) revealed complete reaction of the epoxide groups with 1,6-diaminohexane.
example 3
Example 2 was repeated by replacing 1,6-diaminohexane with 1,4-diaminobutane, 1,5-diaminopentane, 1,7-diaminoheptane, 1,8-diaminooctane, 1,9-diaminononane and 1,10-diaminodecane. In all cases amino-alkyl derivatised agarose matrices were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
| Affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com