Gas sensor and manufacturing method thereof
a technology of gas sensor and manufacturing method, which is applied in the direction of instruments, coatings, material analysis, etc., can solve the problems of headache, dizziness, sickness, headache, nausea, etc., and achieve the effects of high sensitivity, rapid recovery rate, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Gas sensor of the Present Invention and Preparation Thereof
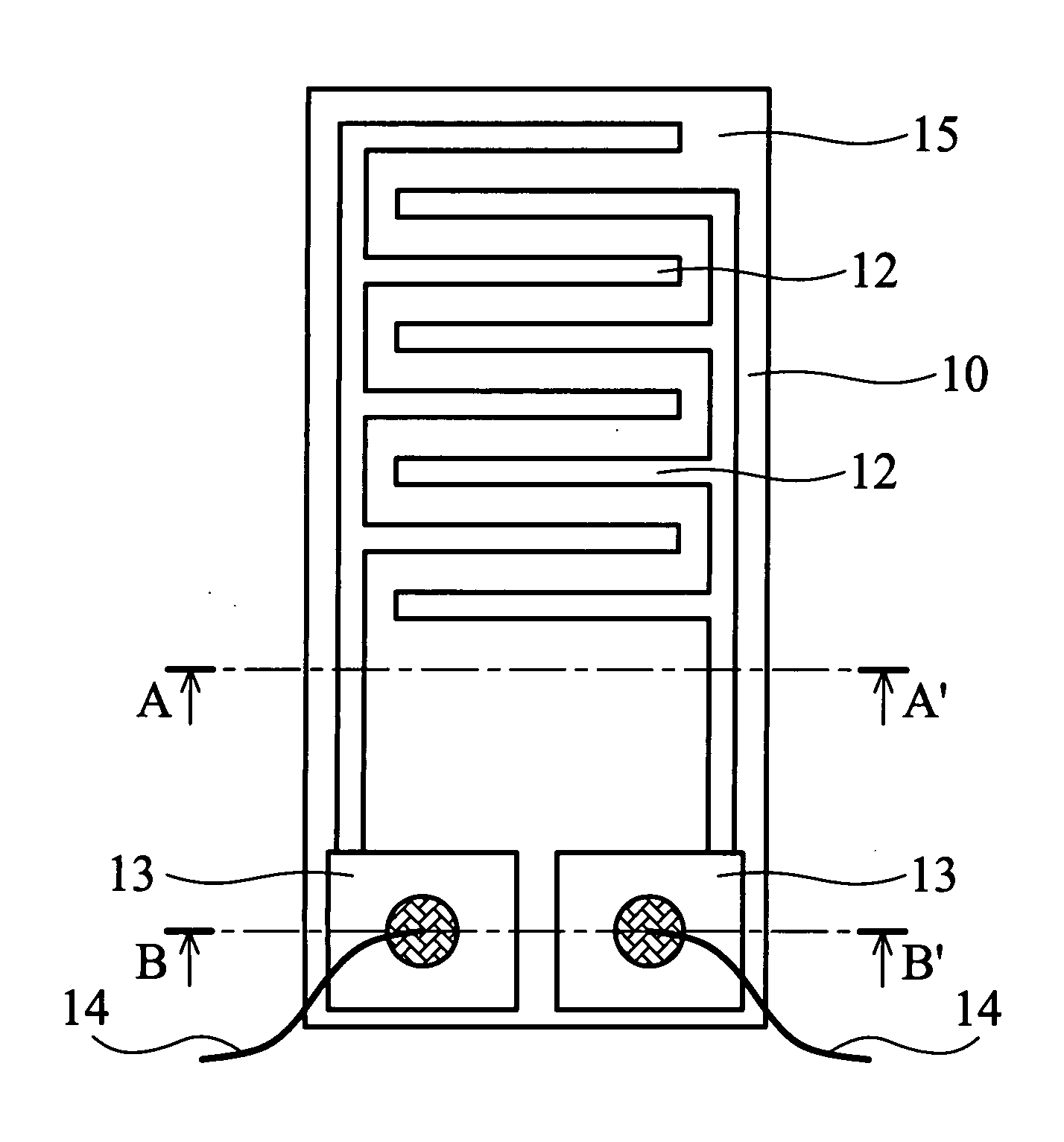
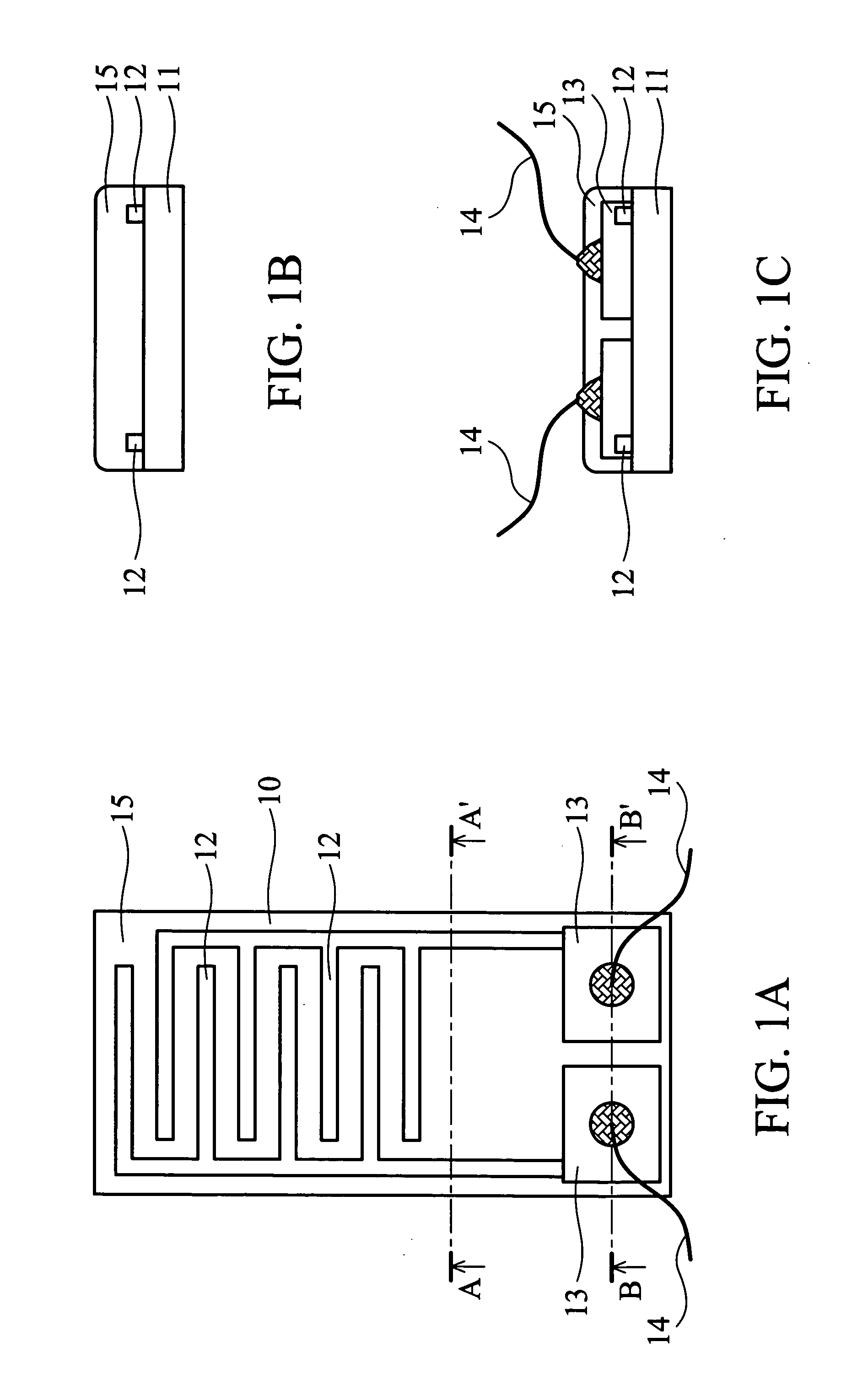
[0036] As shown in FIG. 1A, the gas sensor 10 of the present invention comprises an aluminum oxide ceramic substrate 11 (10×5 mm), with a pair of comb-shaped, gold electrodes 12 thereon. The electrodes do not contact each other. An Ag / Pd layer 13 is coated at the end of the electrodes 12. The gold electrodes 12 and the Ag / Pd layer 13 are formed by screen printing. A SnO2 gas sensing film 15 covers the gold electrode 12.
[0037] First, tin (II) 2-ethylhexanoate was dissolved in 2-ethylhexanoic acid to form an organo-metallic solution at weight percentage 10%. One mg of the single-walled carbon nanotubes (Carbon Nanotechnology Inc., prepared by HiPco process) and 10 g of the organo-metallic solution were mixed. The mixture was vibrated by ultrasonic oscillator for 2 hours to evenly distribute the carbon nanotubes in the solution and form a suspension containing carbon nanotubes. The suspension was then applied to the gold elec...
example 2
Preparation of a Conventional Gas Sensor
[0038] The conventional gas sensor does not contain carbon nanotubes. Preparation of the conventional gas sensor follows:
[0039] Tin (II) 2-ethylhexazoate was dissolved in 2-ethylhexanoic acid to form an organo-metallic solution at weight percentage 10%. The organo-metallic solution was applied to gold electrodes 12 and Ag / Pd layer 13 as shown in FIG. 1. The substrate covered with the solution was then dried at 150° C. for 30 minutes to evaporate the solvent. After that, the substrate was heated in a furnace for half hour with air circulation to dissolve the organo-metallic solution at 500° C., and a microcrystalline and porous SnO2 gas sensing film was formed at a thickness of 5-10 μm.
example 3
Comparison of the Gas Sensors of the Present Invention and the Conventional Gas Sensor
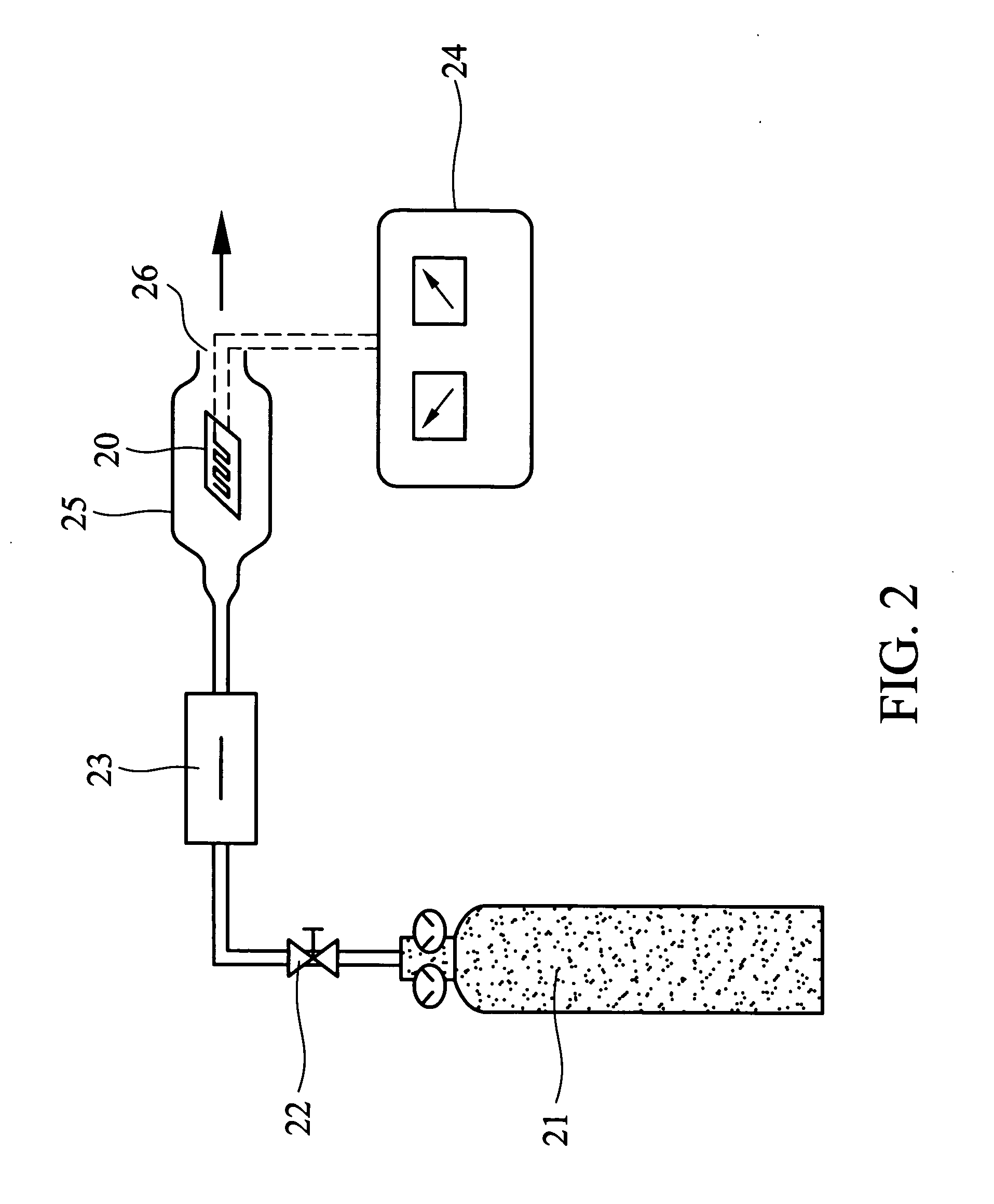
[0040] The schematic diagram of the gas detection apparatus in the experiment is shown in FIG. 2. Before testing, the gas sensing film 15 on the Ag / Pd layer 13 as shown in FIG. 1 was scraped and signal lines 14 were soldered to the Ag / Pd layer 13. The gas sensor 20 of the present invention as shown in FIG. 2 was placed in a glass chamber 25. The input of 1010 ppm NO2 gas 21 was controlled by an intake valve 22 and a mass flow controller 23 100 mL / min. The resistor changes were recorded by LCR meter 24. The outlet of the gas flow is shown as 26 in FIG. 2, with results shown in FIG. 3 and 4.
[0041]FIG. 3 and 4 respectively compare the characteristic curves of the gas sensor of the present invention and the conventional SnO2 gas sensor to 1010 ppm NO2 gas. The coordinates in FIG. 3 and 4 are the same, with mark 1 indicating initialized the input of NO2 and mark 2 stoppage. The right upper plate of FI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com