Transition metal oxide nanowires
a metal oxide nanowire and transition metal oxide technology, applied in the field of transition metal oxide nanowires, can solve the problems of large and expensive magnetic field sensors in their products, poor crystalline quality of highly agglomerated samples, and deformation of the whole crystal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
BaTiO3 Nanowire Synthesis
Three mmol (1.62 g) of barium titanium isopropoxide complex (precursor) is added to 0.3 mmol (0.084 g) of oleic acid (a coordinating ligand) in 10 mL of heptadecane (solvent) (10:1 molar ratio) under inert atmosphere. The reagent mixture is then stirred and heated up to 100° C., and 4 mL of a 3% H2O2 solution is injected into the mixture. After injection, the reaction mixture is heated to 280° C. for 3-12 hours. During this time, the reaction mixture turns from yellow to white as the vigorous bubbling that follows injection subsides. After 3 hours, the reaction is cooled to room temperature. The remaining solution is then washed with methanol and centrifuged to flocculate the nanowires. The supernatant is discarded. The flocculate is then washed with hexane and centrifuged repeatedly to eliminate any remaining heptadecane. After the final centrifugation step, the supernatant is discarded, and the precipitate is dried under vacuum, producing dried nanowire...
example 2
Characterization of BaTiO3 Nanowires
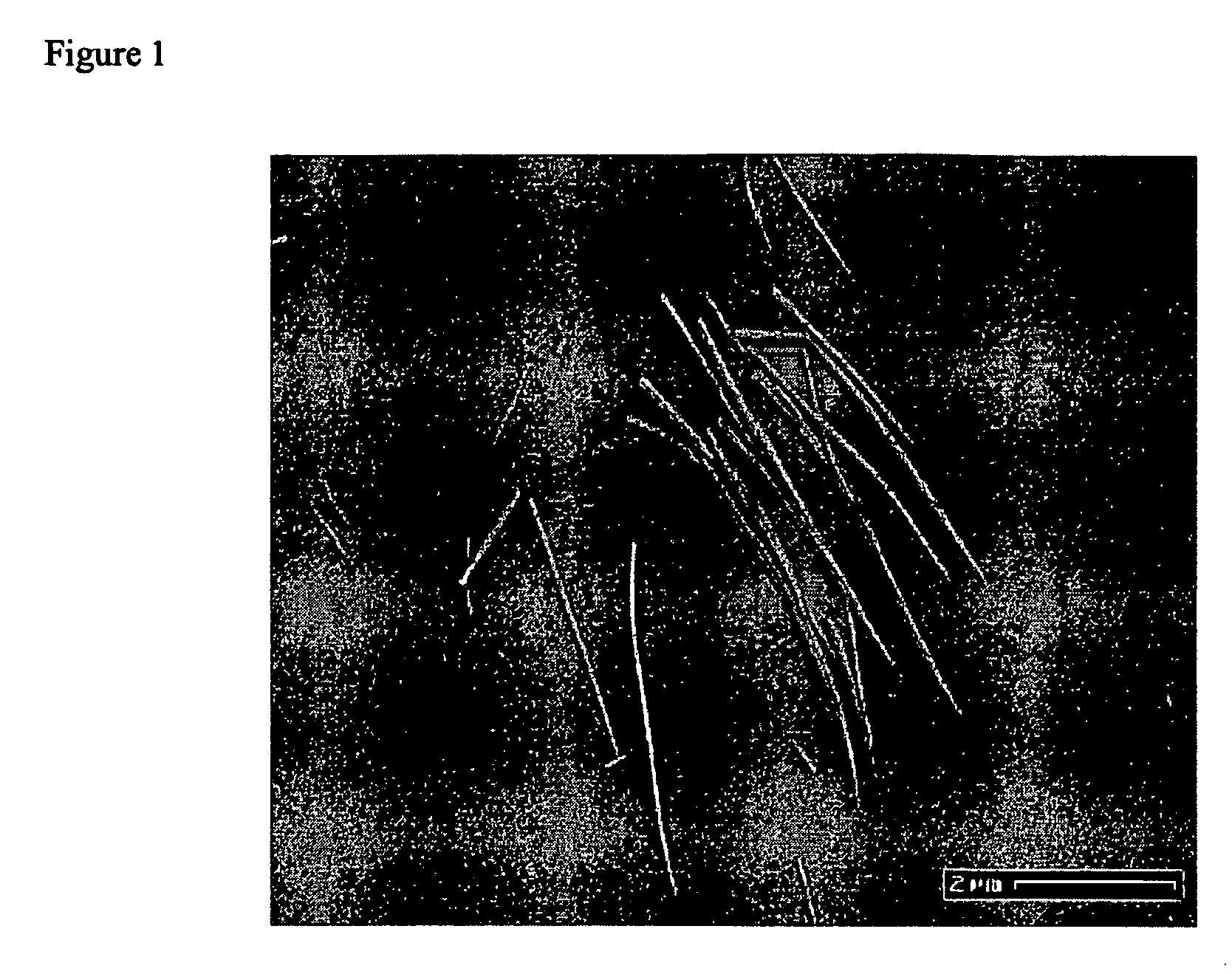
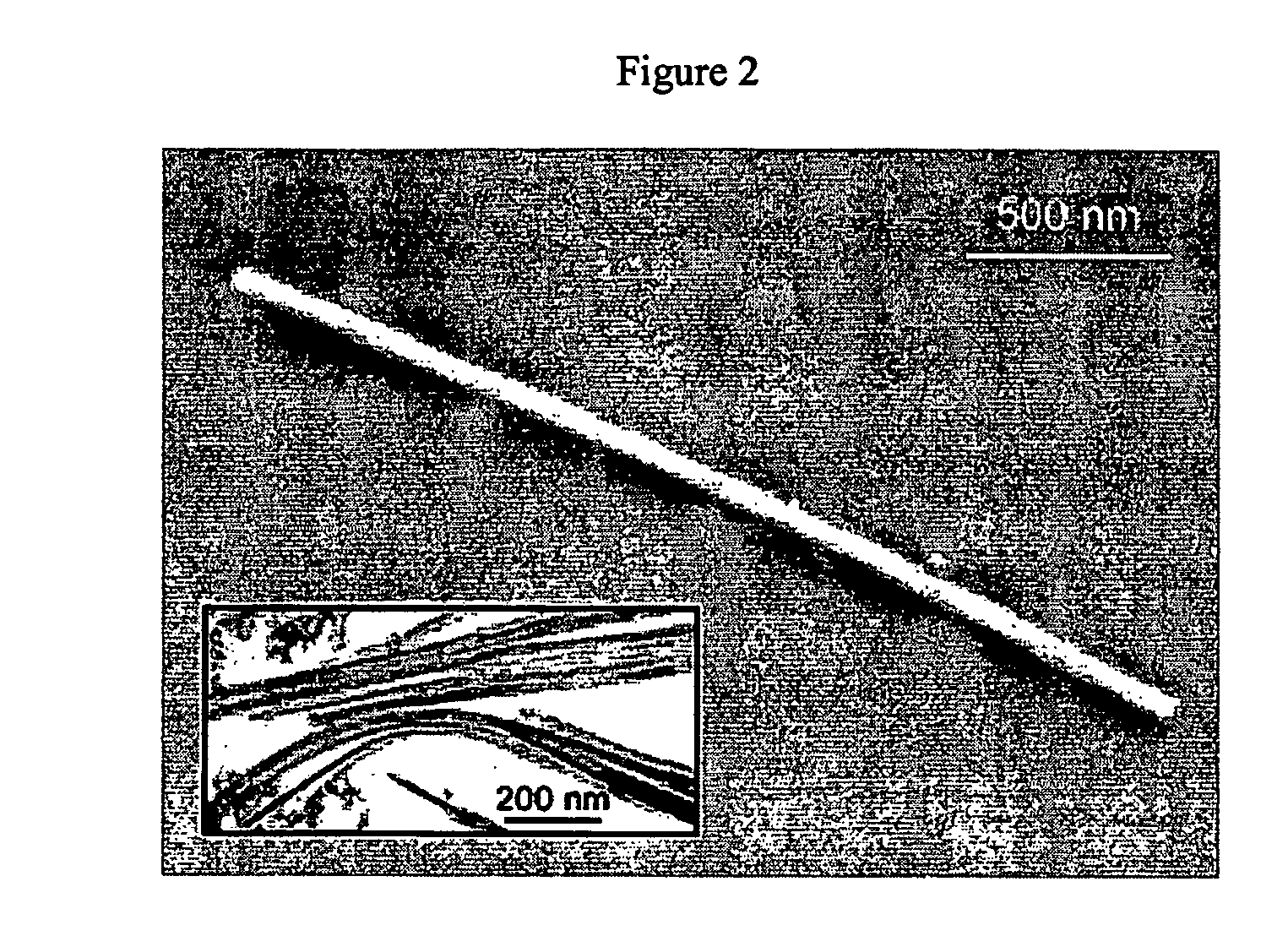
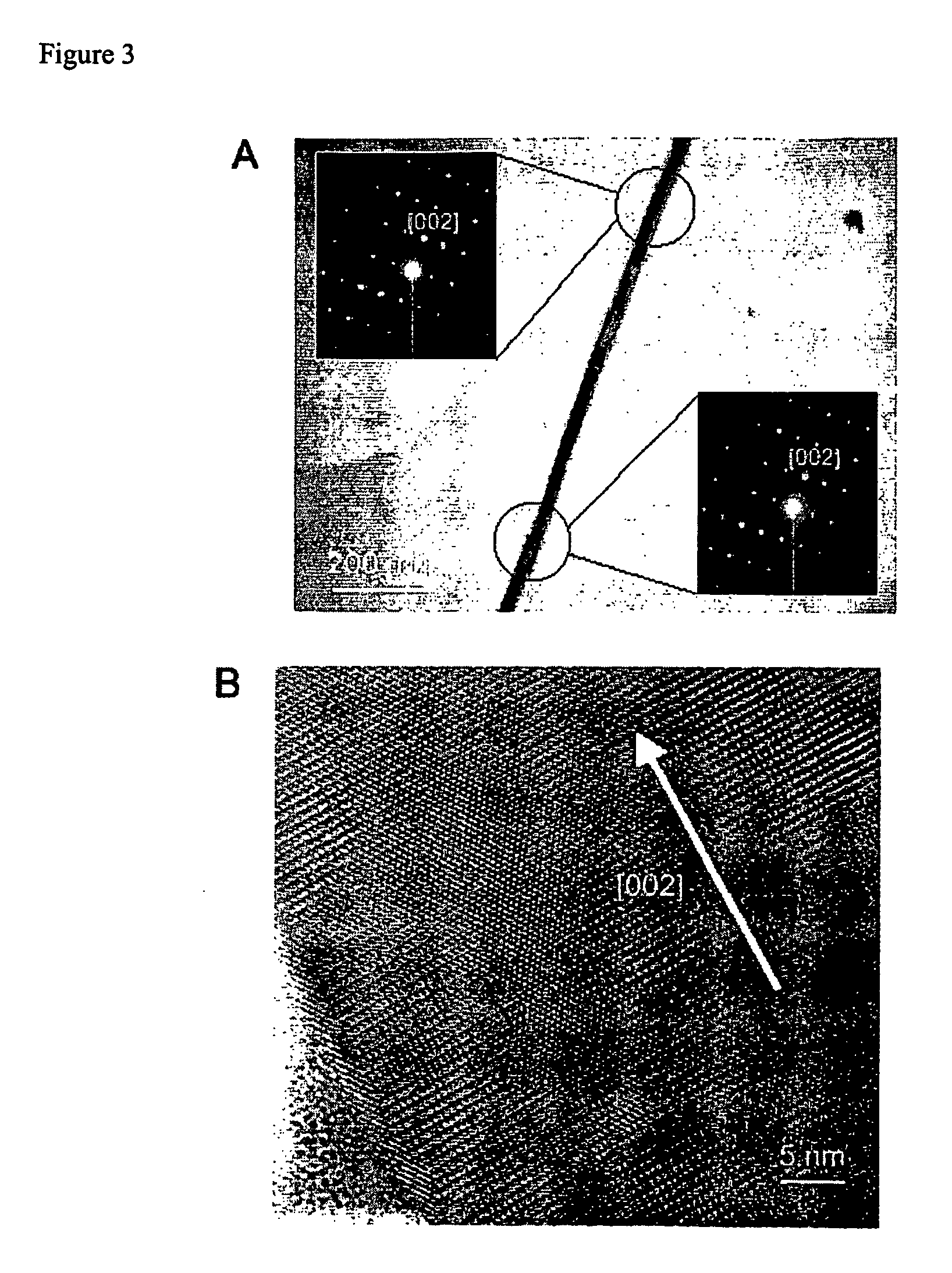
The analysis using scanning electron microscopy (FIGS. 1 and 2), transmission electron microscopy (FIG. 3), X-ray diffractometry (FIG. 4) reveals that the reaction products are single-crystalline BaTiO3 nanowires with diameters from 3 nm to 100 nm and lengths up to >10 μm.
example 3
Preparation of Precursor Barium Alkoxide Precursors
The precursors were synthesized by the following procedure in an inert atmosphere: 65.0 mmol of barium metal was added to a flask containing 112 mL anydrous benzene, 21 ml isopropanol, and 19.5 ml titanium (IV) isopropoxide and stirred vigorously until the added metal was completely dissolved. The solution exhibited a deep purple color within minutes and gradually became white. Once the metal was dissolved, the solution was placed at 4° C. as the precursor precipitated out of the solution. The precipitated precursors were dried overnight, resulting in a fine white powder, with a formula of BaTi(O-iPr)6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com