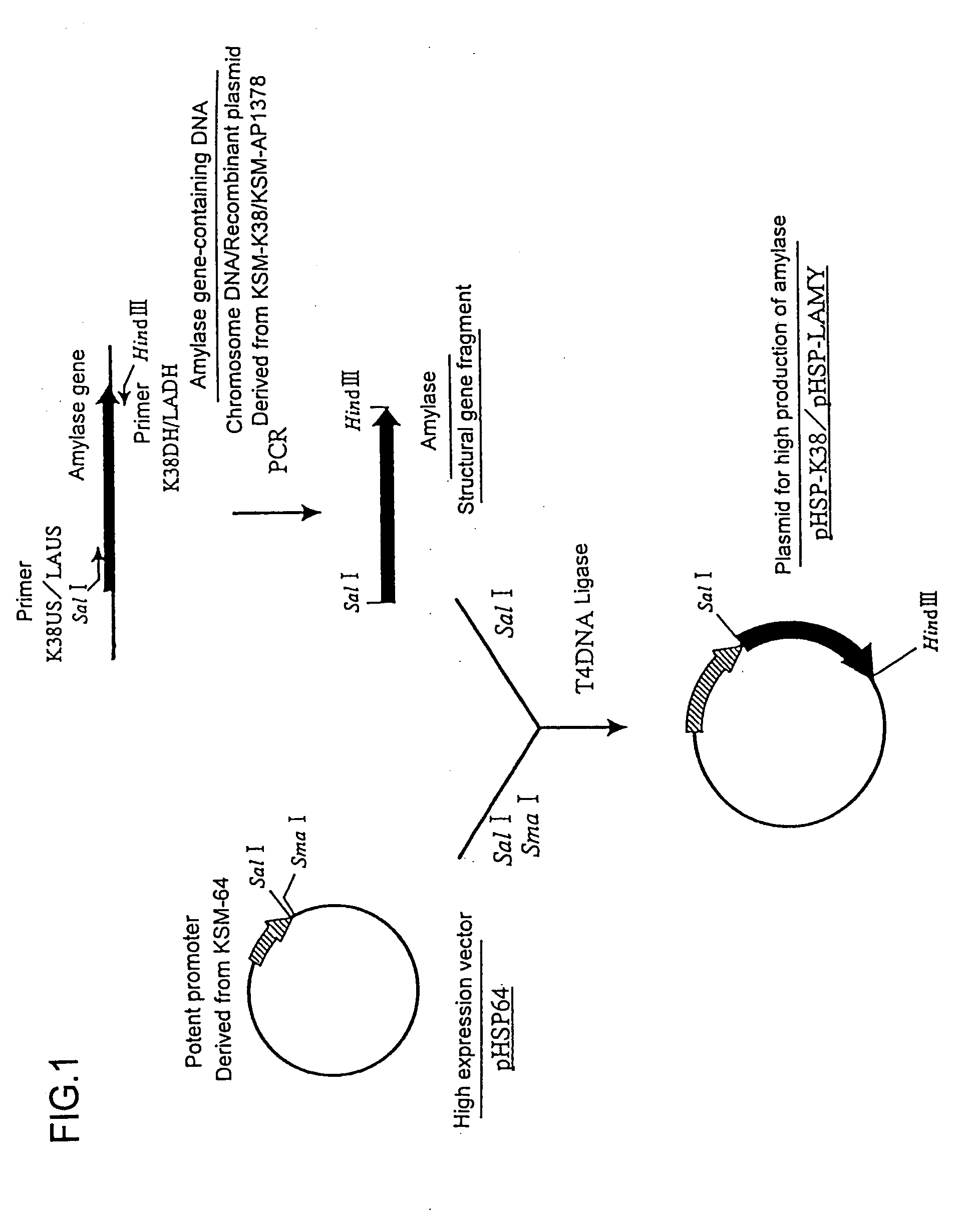
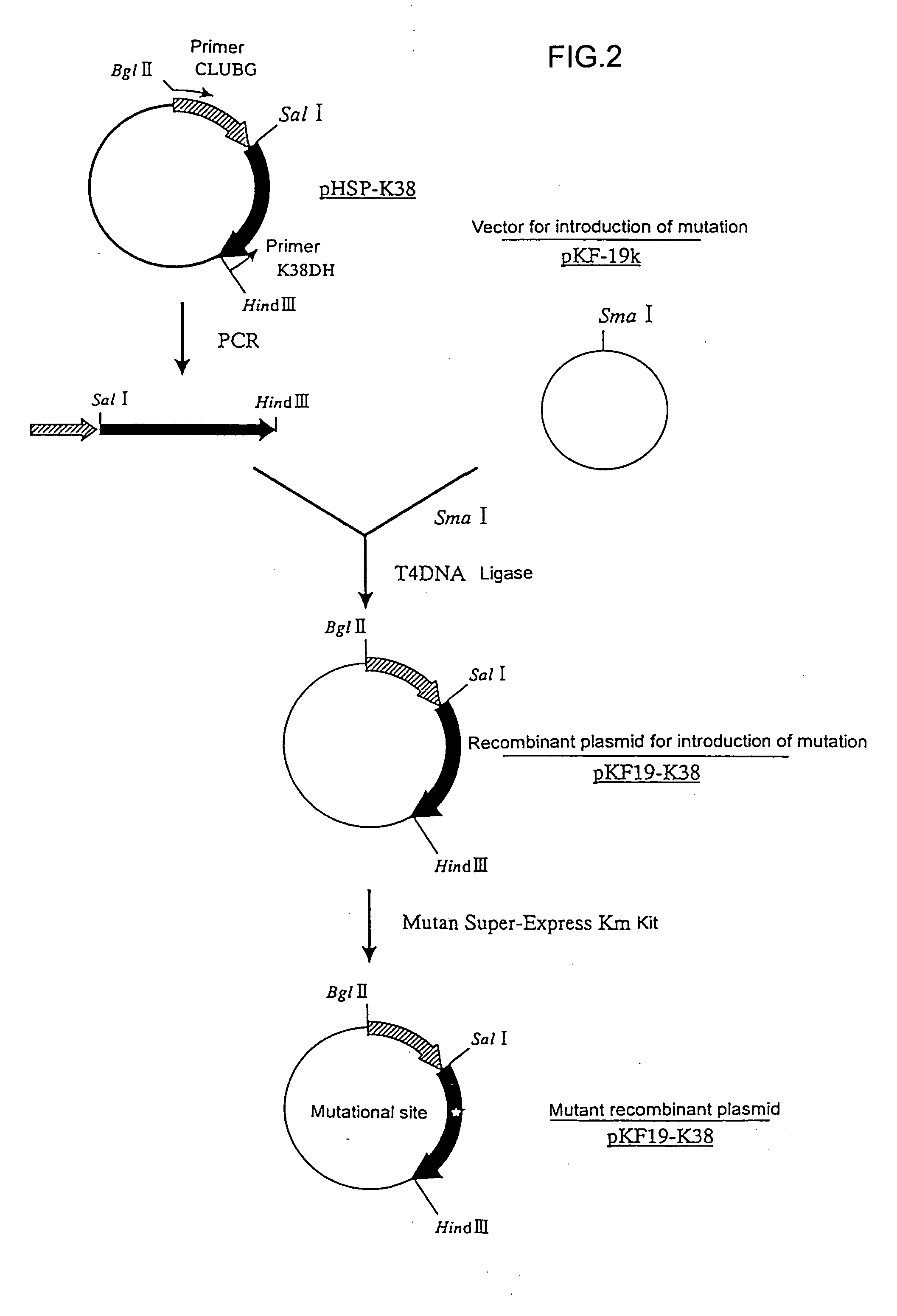
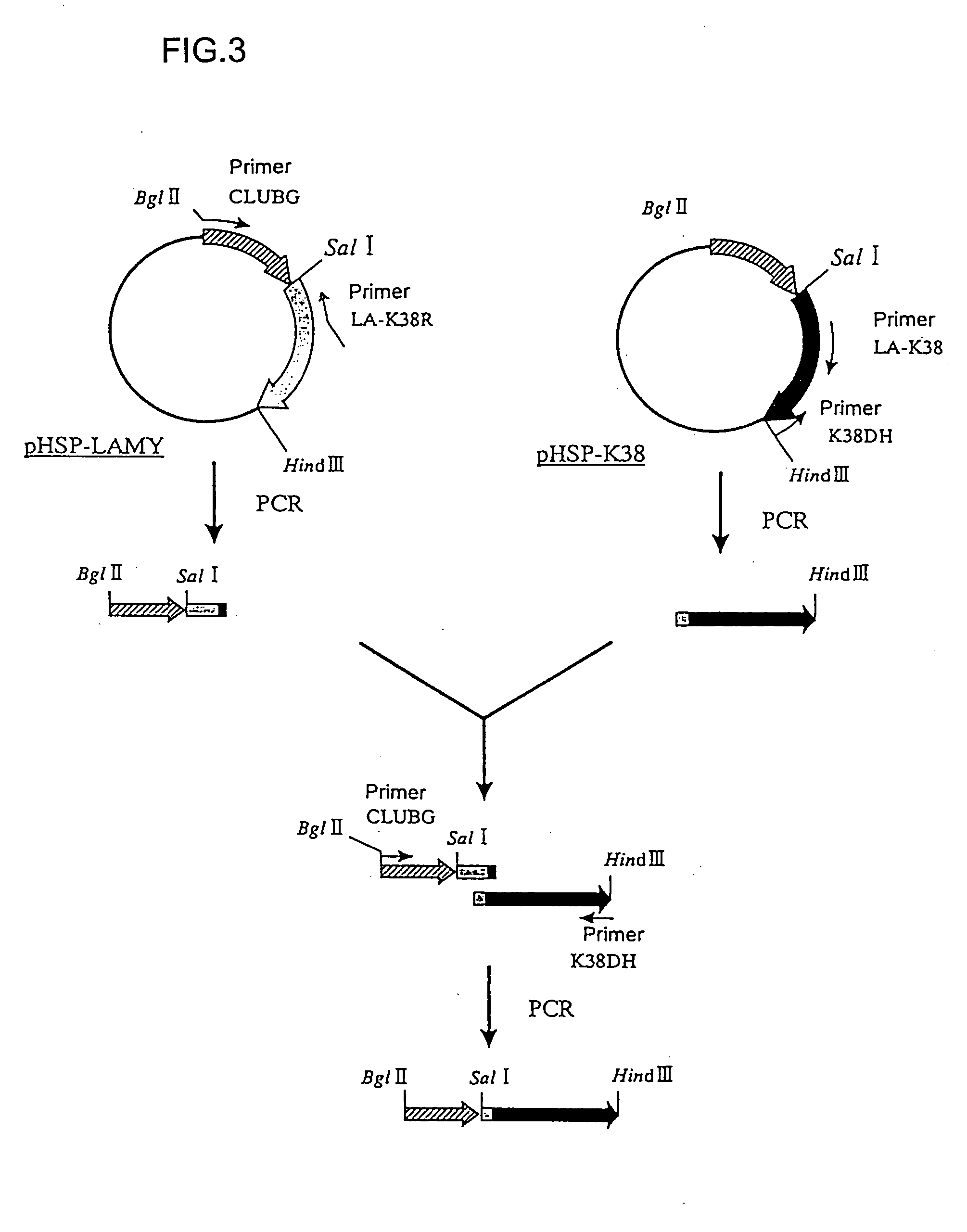
Mutant alpha-amylases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
referential example 1
Screening of Liquefying Alkaline Amylase:
[0036] Soil (about 0.5 g) was suspended in sterilized water and subjected to a heat treatment at 80° C. for 15 minutes. A supernatant of the heat-treated suspension was suitably diluted with sterilized water, and the resultant dilute solution was coated on an agar medium (Medium A) for separation. Culture was then conducted at 30° C. for 2 days to form colonies. Those on the peripheries of which transparent halo based on amylolysis had been formed were screened, and isolated as amylase-producing bacteria. Further, the thus-isolated bacteria were inoculated on Medium B and subjected to aerobic shaking culture at 30° C. for 2 days. After the culture, the resistance performance to a chelating agent (EDTA) of a supernatant centrifugally separated was determined, and its optimum pH was further measured to screen the liquefying alkaline α-amylase-producing bacteria.
[0037]Bacillus sp. KSM-K38 (FERM BP-6946) and Bacillus sp. KSM-K36 (FERM BP-6945)...
reference example 2
Culture of KSM-K38 and KSM-K36 Strains:
[0039] The KSM-K38 or KSM-K36 strain was inoculated on the liquid medium B used in Referential Example 1 to conduct shaking culture at 30° C. for 2 days. The amylase activity (at pH 8.5) of a supernatant centrifugally separated was determined. As a result, these strains had activities of 557 U and 1177 U per liter of the medium, respectively.
referential example 3
Purification of Liquefying Alkaline Amylase:
[0040] Ammonium sulfate was added to the resultant culture supernatant of the KSM-38 strain obtained in Referential Example 2 to 80% saturation. After stirring the resultant mixture, precipitate formed was collected and dissolved in 10 mM Tris-hydrochloride buffer (pH: 7.5) containing 2 mM CaCl2 and dialyzed overnight against the same buffer. The dialyzate thus obtained was passed through a DEAE-Toyopearl 650M column equilibrated with the same buffer and caused to be adsorbed on the column, and the intended enzyme was eluted with the same buffer by 0-1 M gradient of sodium chloride concentration. After the active fraction was dialyzed against the same buffer, an active fraction obtained by gel filtration column chromatography was dialyzed against the above-described buffer, thereby obtaining a purified enzyme which gave a single band on both polyacrylamide gel electrophoresis (gel concentration: 10%) and sodium dodecyl sulfate (SDS) elec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com